Thermal Properties of Matter - Definition, Formulas, Examples, FAQs
The thermal properties of matter are the essential characteristics that govern how substances respond to changes in temperature and energy transfer. In this article, we will explore the thermal expansion of solids, liquids, and gases, specific heat capacity, modes of heat transfer, thermal conductivity, and the laws related to blackbody radiation. By examining these properties, we can gain insights into the behaviour of materials under thermal conditions. This article provides a foundational understanding that is crucial for class 11 students. Students should check the Thermal Properties of Matter class 11 notes to download the pdf containing notes.
This Story also Contains
- 1. Introduction
- Real-life Examples of Thermal Properties of Matter:
- Exam Wise Weightage of Thermal Properties of Matter
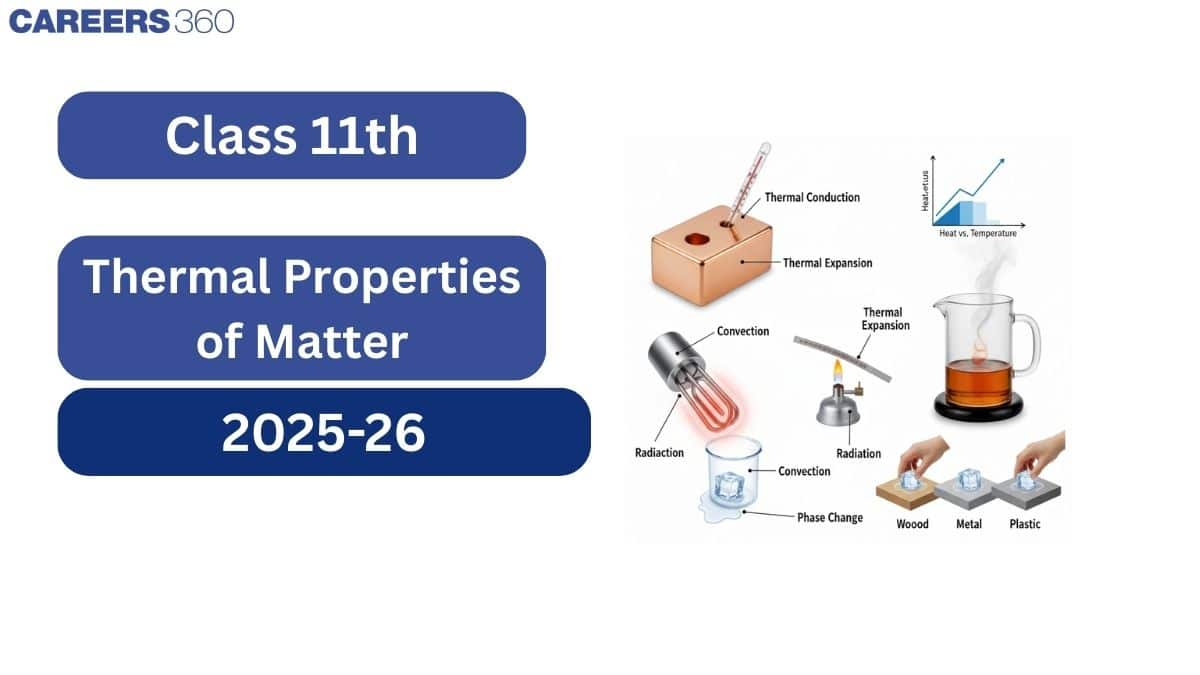
1. Introduction
This chapter explains heat and temperature and how heat flows between bodies. You will learn how heat is measured and why temperature can remain constant during processes like boiling or freezing. Real-life examples, such as heating iron before fitting it on a wheel or beach wind changes, illustrate these concepts.
2. Temperature and Heat
Temperature is a measure of the hotness or coldness of a body. It determines the direction of heat flow.
Heat is a form of energy transferred between bodies due to a temperature difference.
3. Measurement of Temperature
Temperature is measured using a thermometer, based on a property that changes with temperature (like length, volume, resistance, or pressure).
Common thermometers: Mercury thermometer, Alcohol thermometer, Gas thermometer, Resistance thermometer.
Temperature scales: Celsius (°C), Fahrenheit (°F), and Kelvin (K).
In physics, the Kelvin scale is used as the standard (SI unit).
4. Ideal Gas Equation and Absolute Temperature
For an ideal gas, the relation between pressure ( P ), volume ( V ), and temperature ( T ) is given by the ideal gas equation:
$
P V=n R T
$
where $n=$ number of moles, $R=$ universal gas constant.
Absolute temperature is measured on the Kelvin scale, where 0 K is the lowest possible temperature (absolute zero).
5. Thermal Expansion
Thermal expansion is the increase in the size (length, area, or volume) of a body when its temperature rises.
Types:
Linear expansion – Increase in length.
Areal expansion – Increase in surface area.
Volumetric expansion – Increase in volume.
6. Specific Heat Capacity
Specific heat capacity (c) of a substance is the amount of heat required to raise the temperature of $\mathbf{1}$ kg of the substance by 1 K (or $1^{\circ} \mathrm{C}$ ).
Formula:
$
Q=m c \Delta T
$
7. Calorimetry
Calorimetry is the science of measuring the amount of heat exchanged in physical and chemical processes.
Based on the principle of conservation of energy:
Heat lost by hot body $=$ Heat gained by cold body
A device called a calorimeter (usually made of copper with insulation) is used for such measurements.
8. Change of State
Change of state means conversion of matter from one form (solid, liquid, gas) to another by absorbing or releasing heat.
Common changes:
- Melting (solid $\rightarrow$ liquid)
- Freezing (liquid $\rightarrow$ solid)
- Vaporisation (liquid $\rightarrow$ gas)
- Condensation (gas $\rightarrow$ liquid)
- Sublimation (solid $\leftrightarrow$ gas)
9. Heat Transfer
Heat can flow from a hotter body to a colder body by three main methods:
Conduction – Transfer of heat through a material without actual movement of particles. (e.g., heating one end of a metal rod).
Convection – Transfer of heat by actual movement of fluid (liquid or gas). (e.g., sea breeze, boiling water).
Radiation – Transfer of heat in the form of electromagnetic waves, without any medium. (e.g., heat from the Sun).
10. Newton's Law of Cooling
Newton's law of cooling states that the rate of loss of heat of a body is directly proportional to the temperature difference between the body and its surroundings, provided this difference is small.
$
\frac{d Q}{d t} \propto\left(T-T_{\mathrm{sur}}\right)
$
where $T=$ temperature of body, $T_{\text {sur }}=$ temperature of surroundings.
Real-life Examples of Thermal Properties of Matter:
Railway tracks and bridges – Gaps are left to allow for thermal expansion in hot weather.
Sea and land breeze – Caused by convection of air due to the temperature difference between land and sea.
Cooking utensils – Metals are used for good conduction of heat, handles are plastic/wood (bad conductors).
Thermos flask – Uses the idea of reducing conduction, convection, and radiation to keep liquids hot or cold.
Exam Wise Weightage of Thermal Properties of Matter
| Exam | Weightage | Remarks |
|---|---|---|
| JEE Main | Usually 1 question | Topics like calorimetry, expansion, and heat transfer are common. |
| JEE Advanced | 1 question | Often conceptual or application-based (e.g., Newton’s law of cooling, calorimetry). |
| NEET (Physics) | 1question | mostly direct and formula-based (e.g., specific heat, heat transfer). |
NCERT Solutions Subject-wise link:
Frequently Asked Questions (FAQs)
Heat is transferred in gas through convection.
The SI unit of thermal conductivity is $\mathrm{W} / \mathrm{m} \cdot \mathrm{K}$ (Watts per meter Kelvin).
Temperature is defined as the degree of hotness or coldness of a body and its measurement is thermometry.
The three modes of heat transfer are convection, conduction and radiation.
The SI unit of heat is Joule (J)
