Amines - Notes, Topics, Formula, Books, FAQs
What is the first thing that comes to your mind when you are bored or sleepy? An energy drink or maybe a soda? Do you know that coffee contains caffeine, an alkaloid with amine groups? They block sleep-inducing receptors in the brain. This is how active amines are in our daily lives. Amines are organic molecules created by replacing one or more of ammonia’s hydrogen atoms with alkyl or aromatic groups. The nitrogen atom keeps a lone pair, which gives amines mild basic properties. They exist naturally in proteins, vitamins, hormones, and amino acids, and are also synthesized for dyes, medicines, polymers, and water treatment.
This Story also Contains
- Important Topics - Organic compounds Containing Nitrogen
- Overview Of The Chapter
- How To Prepare For Amines?
- Prescribed Books
- Study Links for Amines
- Amines in Different Exams:
- Subject Wise Resources of NCERT
- Amines Previous Year Question and Answers
- Conclusion:
.jpg)
Amines are vital organic compounds found in many substances, from neurotransmitters and hormones to medicines and industrial chemicals. They are widely found in Proteins, Vitamins, and hormones, and are derivatives of ammonia, where one or more hydrogen atoms are replaced by alkyl or aryl groups. Small amines often smell fishy, are water‑soluble if their carbon chains are short, and tend to have higher boiling points when they can hydrogen bond. Their basic strength depends on the substituents and possible resonance effects in aromatic structures. This chapter covers amine structures, synthesis methods, properties, reactions, and uses in real‑world applications.
Important Topics - Organic compounds Containing Nitrogen
Organic nitrogen-containing compounds include a variety of classes like amines, amides, nitriles, nitro compounds, amino acids and peptides, diazonium salts, and heterocyclic nitrogen compounds (alkaloids, imidazoles, etc.).
Key topics covered in this chapter of Chemistry are classification and naming of amines, methods like Gabriel synthesis and Hofmann degradation, reactions such as nitration, reduction, diazotization and azo coupling, and biological roles of amino acids and heterocycles.
Preparation of Amines:
Amines are the class of organic compounds produced from ammonia NH₃ by replacement of one or more hydrogen atoms with either alkyl or aryl groups. They are classified into three categories: primary, secondary, and tertiary amines. There are various preparations of amines such as Curtuis reaction, Schmidt reaction, loosen reaction, and many more.
Preparation And Properties Of Aromatic Nitrocompounds:
Nitro compounds are highly polar and hence soluble in polar solvents. They usually have higher boiling points than the non-nitro derivatives since there are strong intermolecular forces. Preparation of aromatic nitro compounds is the process of the nitration of aromatic hydrocarbons. This is usually an electrophilic substitution where an aromatic compound reacts with a nitrating agent; commonly, this is a mixture of concentrated nitric acid and sulfuric acid.
Test For Amines:
There are various tests for amines such as when any primary amine(aliphatic or aromatic) is heated with chloroform and alcoholic potassium hydroxide solution, isocyanide(carbylamine) is formed which has a very unpleasant smell. This test is called the carbylamine test or isocyanide test.
Basicity Of Amines:
Basicity is defined as either the acceptance of protons or the donation of electron pairs by a substance. Amines are classified according to the number of groups attached to the nitrogen atom: primary, secondary, and tertiary amines. And the basic nature is due to the presence of an unshared pair of electrons on a nitrogen atom. This lone pair of electrons is available for the formation of a new bond with a proton or Lewis acids.
Overview Of The Chapter
In this chapter, there are various important topics that you must understand completely:
Structure Of Amines:
In amines, the nitrogen is in $s p^3$ hybridisation with 3 sigma bonds and 1 lone pair of electrons. Amines possess the tetrahedral geometry but the bond angle in its structure is always less than 109.50 because nitrogen atom has a lone pair of electrons which reduces its bond angle.
.png)
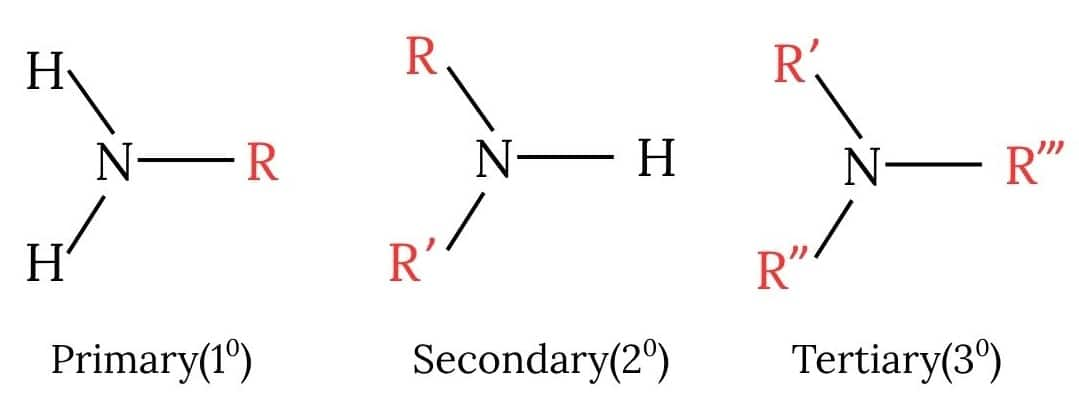
Classification Of Amines:
Amines can be classified into three categories as follows, depending on the number of alkyl or aryl groups attached to the nitrogen atom:
Preparation Of Amines:
Amines can be prepared from the following methods:
- Reduction Of Nitro Compounds: In this reaction, the nitro compounds are reduced by the ($\mathrm{H}_2$ / Pd) reagent to amines.
.png)
- Reduction of Nitriles: In this reaction, nitriles are reduced to amines by another equally strong reducing agent i.e ($\mathrm{H}_2$/ Ni).
.png)
- Hoffmann Bromamide Degradation Reaction: In this reaction, the amide is treated with bromine in the presence of an aqueous solution of NaOH. This reaction converts amide to a primary amine with 1 carbon less than the amide.
Physical Properties
- Lower aliphatic amines are gases with a fishy smell and higher aliphatic amines are liquid.
- Lower aliphatic amines are soluble in water because of the capability of hydrogen bonding with the water molecules.
- Amines are soluble in organic solvents as well such as alcohol, ether, benzene, etc.
- The boiling point of amines follows the order below:
Primary > Secondary> Tertiary - Intermolecular interaction of amines is more prevalent in primary amines than in secondary amines and this interaction is absent in tertiary amines.
Chemical Reactions
1. Alkylation Of Amines: In this reaction, amines are reacted with alkyl halides as shown below in fig. The product formed in this reaction is 10 higher amine.
.png)
2. Acylation Of Amines: In this reaction, anhydrides are reacted with amines and the product formed in this reaction is amide.
.png)
3. Carbylamine Reaction: In this reaction, primary amines are reacted with chloroform in the presence of potassium hydroxide and the product formed is isocyanides as shown in the figure given below:
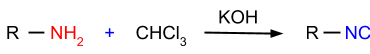
4. Reaction with Nitrous Acid: In this reaction, amines are reacted with nitrous acid and form diazonium salt or alcohol, depending on which type of amine is reacting with nitrous acid.
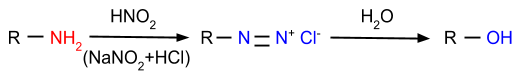
Applications
In the real world, amines are largely used for many applications as follows:
- Methylamines are used for making various agricultural products such as herbicides, insecticides, miticides, etc.
- There are various kinds of amines such as MEA, DEA, DGA, and others are used in industries for removing carbon dioxide and hydrogen sulfide.
- Aromatic amines are used for the production of dyes.
- Amines are used in various kinds of drugs such as chlorpheniramine, ephedrine, amitriptyline, etc.
How To Prepare For Amines?
1. The "Amines" chapter is theoretical and focuses on understanding reaction mechanisms rather than memorizing formulas. Here's how to prepare effectively:
2. Review Basic Organic Chemistry Concepts: Start with Unit 12 of the NCERT Class 11 Part II textbook to strengthen your foundational knowledge.
3. Understand Key Reactions: Focus on important reactions like Gabriel Synthesis, Hofmann Rearrangement, and reduction of nitro compounds.
4. Practice Reaction Mechanisms: Regularly practice drawing and explaining the steps involved in these reactions to reinforce your understanding.
5. Utilize Visual Aids: Refer to diagrams and reaction schemes to visualize the processes and enhance memory retention.
6. Review and Revise Regularly: Consistent revision is key to retaining information and gaining proficiency in the subject.
Prescribed Books
For this chapter, first, the NCERT book is best for initial-level preparation as well as for board exams. Now, after this, if you want to prepare for competitive exams like JEE and NEET, then these are the best books for you - Morrison and Boyd and R.K Gupta by Arihant publication. Meanwhile, in the preparation, you must continuously give the mock tests for the depth of knowledge. Our platform will help you with a variety of questions for deeper knowledge with the help of videos, articles, and mock tests.
Study Links for Amines
In this section, you’ll find the different resources for studying the chapter Amines. These study links are designed to support effective learning and quick revision. They include detailed notes, NCERT exemplar questions, and NCERT solutions to all the questions.
Amines in Different Exams:
Amines is commonly asked in board and competitive examinations with focus on classification, basicity, preparation methods, and important reactions of amines.
| Exam Name | Focus Area | Common Topics Asked | Preperation Tips |
| CBSE Board | Conceptual understanding | Classification, preparation, reactions | Revise NCERT reactions and examples |
| JEE Main | Concept application | Basicity of amines, reactions | Practise MCQs and conversions |
| JEE Advanced | Analytical & mechanism-based | Reaction mechanisms, Hoffmann bromamide | Focus on detailed mechanisms |
| NEET | Basic concepts | Uses, reactions, IUPAC naming | Study NCERT line by line |
| State Board Exams | Theory-oriented | Definitions, preparation methods | Learn key reactions and properties |
| Chemistry Olympiads | Advanced application | Reaction mechanisms, comparative basicity | Practise advanced organic problems |
Subject Wise Resources of NCERT
This section provides organised study materials and useful links for each subject to support effective learning and exam preparation.
Amines Previous Year Question and Answers
Some of the questions that have come in past years from the chapter are given below. By referring to the previous year questions, students can understand this chapter in a better way.
Question 1: 
The ratio of number of oxygen atoms to bromine atoms in the product $\mathrm{Q}$ is ______ $\times 10^{-1}$.
Answer:

Hence, the answer is (15).
Question 2: An amine $(\mathrm{X})$ is prepared by ammonolysis of benzyl chloride. On adding p-toluenesulfonylchloride to it the solution remains clear. Molar mass of the amine $(\mathrm{X})$ formed is _______$\mathrm{g} \mathrm{mol}^{-1}$.
(Given molar mass in gmol $^{-1} \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{O}: 16, \mathrm{~N}: 14$ )
Answer:
Molar Mass of $(\mathrm{X})$ is $287 \mathrm{~g} \mathrm{~mol}^{-1}$
Hence, the answer is the option (1).
Practice more questions from the link given below:
Conclusion:
The "Amines" chapter explores nitrogen-containing organic compounds, focusing on their structure, classification, and reactions. Amines are vital in pharmaceuticals, agriculture, and industrial chemistry. Understanding their properties is crucial for applications like drug synthesis and pesticide formulation.
In competitive exams, amines hold a weightage of approximately 5% in NEET 2025 . Mastery of this chapter enhances problem-solving skills and conceptual clarity, benefiting students aiming for top medical and engineering institutions.
Frequently Asked Questions (FAQs)
Primary aliphatic amines react with nitrous acid to release nitrogen gas and form alcohols. Aromatic primary amines form diazonium salts, which can undergo coupling reactions to produce azo dyes.
Primary and secondary amines can form hydrogen bonds due to the presence of N-H bonds, leading to higher boiling points. Tertiary amines lack N-H bonds and thus cannot form hydrogen bonds, resulting in lower boiling points.
The Gabriel phthalimide synthesis is a method to prepare primary amines by nucleophilic substitution, providing a way to introduce amine groups into organic molecules.
Lower molecular weight amines are soluble in water due to hydrogen bonding. As the hydrophobic alkyl chain length increases, solubility decreases.
Nitrogen-containing compounds are chemical compounds that comprise nitrogen atoms bonded to other elements, such as carbon, hydrogen, oxygen, and
The solubility of amines in water varies based on their structure. Generally, lower molecular weight amines (especially primary and secondary) are soluble in water due to their ability to form hydrogen bonds. As the hydrophobic carbon chain length increases, solubility tends to decrease.
The key difference is that amines are derivatives of ammonia with an attached alkyl or aryl group, whereas amides are formed by the reaction of carboxylic acids with amines, where the nitrogen atom is bonded to a carbonyl group (C=O). Amides generally have different properties and reactivity than amines.
Yes, amines can participate in hydrogen bonding due to the presence of the nitrogen atom, which has a lone pair of electrons. This ability can significantly affect the physical properties of amines, such as their boiling points and solubility in water.
The most common types include:
- Amines: Compounds derived from ammonia with one or more hydrogen atoms replaced by hydrocarbon groups.
- Amino acids: Organic compounds that combine to form proteins, containing both an amino group (–NH2) and a carboxyl group (–COOH).
- Nitriles: Compounds containing a cyano group (–C≡N).
- Nitrosamines: Compounds formed by the reaction of nitrites with amines, some of which can be carcinogenic.
- Nitrates and nitrites: Inorganic compounds that contain the nitrate (NO3−) or nitrite (NO2−) ion.
Questions related to
On Question asked by student community
Correct Answer: Phthalaldehyde
Solution : The correct answer is Phthalaldehyde.
Phthalaldehyde is a dialdehyde in which two formal groups are attached to adjacent carbon centres on a benzene ring. It forms a fluorescent conjugation product with primary amines. It is used as a disinfectant, mainly for dental and medical

