Ammonium Phosphate - Formula, Properties, Uses, FAQs
Have you ever wondered how certain compounds, like ammonium phosphate, play a crucial role in agriculture and industrial chemistry? How is it formed ? What makes ammonium phosphate a popular fertilizer for plants? You will get these answers by reading this article on ammonium phosphate. Ammonium phosphate $\left(\mathrm{NH}_4\right)_3 \mathrm{PO}_4$ is a salt of ammonia and phosphoric acid. It is also known as ammonium orthophosphate. It consists of ammonium cations and phosphate anions ($\mathrm{NH}_4{ }^{+}$ and $\mathrm{PO}_4^{2-}$). It is a water-soluble salt, and if the aqueous solution is boiled, it liberates ammonia.
This Story also Contains
- Formula Of Ammonium Phosphate
- Diammonium Phosphate Formula
- Uses of Ammonium phosphate (Mono Ammonium Phosphate Uses)
- Some Solved Examples
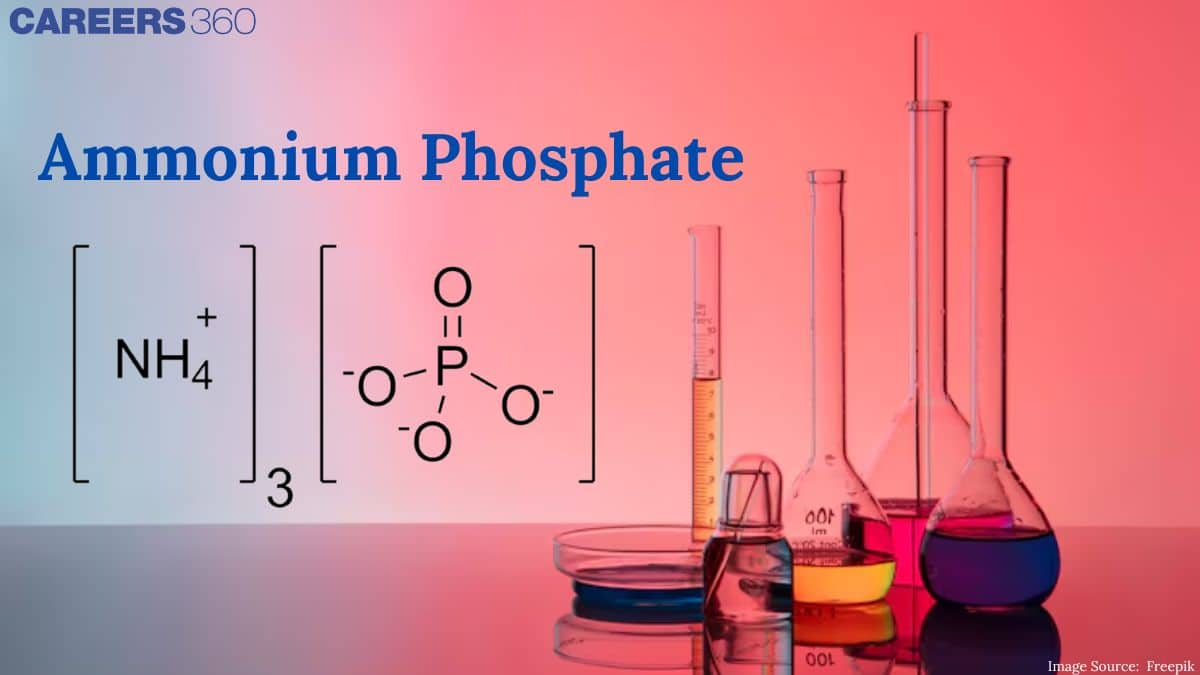
Formula Of Ammonium Phosphate
The molecular formula of ammonium phosphate is- $\left(\mathrm{NH}_4\right)_3 \mathrm{PO}_4$
Ammonium Phosphate as Fertilizer
Ammonium phosphates can be applied to soil as nitrogen and phosphorus-containing fertilizer either directly, or as a solution, or in a suspension form. The distribution pattern depends on the proportion of insoluble phosphates present in the soil. Ammonium orthophosphates are preferred to a generic class of phosphorus fertilizers. These are manufactured by reacting anhydrous ammonia with orthophosphoric acid or superphosphoric acid on a large scale. These are prepared either in solid or liquid form.
There are two main types of ammonium phosphate: monoammonium phosphate (MAP) and diammonium phosphate (DAP). These two major types of ammonium phosphate are interconvertible by changing ammonia or phosphoric acid as per requirement.
Mono-ammonium phosphate, or Ammonium hydrogen phosphate, is manufactured by reacting ammonia with phosphoric acid in a reactor and consequently centrifuging and drying in a rotary dryer in the manufacturing industry. The manufacturing of diammonium phosphate requires a two-stage reactor system in order to avoid loss of ammonia. Thereafter, the granulation followed by neutralization processes is completed in a rotary dryer, which is heated by a furnace using fuel.
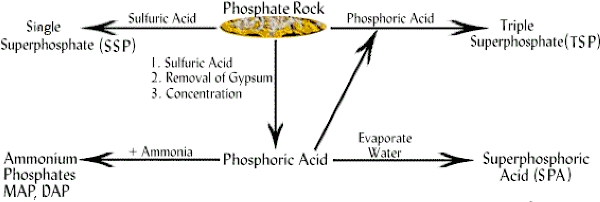
Chemical Formula Of Ammonium Hydrogen Phosphate
The molecular formula of Ammonium hydrogen phosphate or monoammonium phosphate is- $\mathrm{NH}_4 \mathrm{H}_2 \mathrm{PO}_4$
Also read -
Diammonium Phosphate Formula
The molecular formula of Diammonium phosphate is- $\left(\mathrm{NH}_4\right)_2 \mathrm{HPO}_4$
Two grades of ammonium phosphate are available
- Monoammonium phosphate (MAP): Anhydrous ammonia is added to liquid phosphoric acid to produce monoammonium phosphate (MAP). It is a fertilizer or can be considered as a fertilizer intermediate with high P2O5 content, i.e., almost 55%. Its nitrogen content is about 11-12%.
Properties:
- Ammonium phosphate molecular weight (molar mass of (NH4)3PO4/molecular mass of ammonium phosphate/ ammonium phosphate molar mass): Molecular mass of ammonium phosphate is- 115.03gm/mole
- Appearance: White crystal
- Odour: Odourless
- Melting point: 1900 °C
- Density: 1.803gm/mL
- Solubility: Moderately soluble in water
- pH: 4-4.5
- These fertilizers are highly soluble in water and react fast in the soil to give nitrogen and phosphorus in a chemical combination.
- Pure ammonium phosphates are used mainly as liquid fertilizers since it is completely soluble in water.
- Diammonium phosphate is unstable at temperatures above 1500˚C, whereas monoammonium phosphate is stable even at higher temperatures than 1500˚C.
- Monoammonium and diammonium phosphates usually form a part of concentrated compound fertilizer and are used instead of pure states.
2. Diammonium phosphate (DAP): It is manufactured by adding more ammonia to phosphoric acid. In diammonium phosphate (DAP) nitrogen content is about 16 to 18% and the phosphorus content is about 20 to 21 % phosphorus (46% P2O5).
Diammonium phosphate molecular weight
The molecular mass of diammonium phosphate is- 132.06 g/mol
Atomicity of ammonium phosphate
The atomicity of the ammonium phosphate molecule is 20
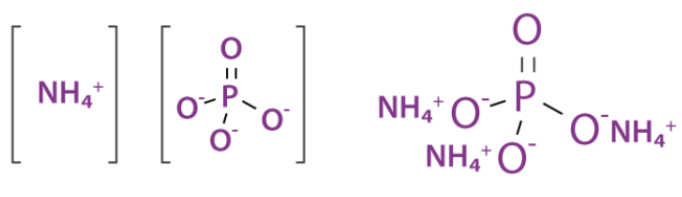
Manufacture of ammonium phosphate
Raw Materials: Ammonia, Phosphoric acid
Reactions
$\mathrm{NH}_3+\mathrm{H}_3 \mathrm{PO}_4 \rightarrow \mathrm{NH}_4 \mathrm{H}_2 \mathrm{PO}_4$
$\mathrm{NH}_3+\mathrm{NH}_4 \mathrm{H}_2 \mathrm{PO}_4 \rightarrow\left(\mathrm{NH}_4\right)_2 \mathrm{HPO}_4$
Sources of raw material
Ammonia can be synthesized by the Haber process
Phosphoric acid can be manufactured by electric arc furnace, blast furnace, or wet process
|
Related Topics, |
Ammonium phosphate can be produced on a large scale in the industry by following two principal steps-
a) Neutralization
b) Granulation
Neutralization
Quantities of phosphoric acid and ammonia required during manufacturing in the neutralization step are different for monoammonium phosphate (MAP) and diammonium phosphate (DAP). In the production mono ammonium phosphate, the ammonia to phosphoric acid ratio should be maintained at 0.6 in the neutralizer and 1.0 in the granulator. While for diammonium phosphate, the ratios of ammonia to phosphoric acid should be maintained at 1.4 in the neutralizer and 1.0 in the granulator.
- Step 1: Phosphoric acid is added to ammonia in the first of three continuous mixed reactors. In the first neutralizer, beneath the slurry level, anhydrous ammonia is added in a particular amount so that almost 80% neutralization can occur in the 1st tank.
- Step 2: Further ammonia is added in the 2nd and 3rd neutralizer to obtain conversion of diammonium phosphate with higher nitrogen content. The heat obtained from the exothermic reaction inside the tank is enough to heat the slurry nearly to the boiling point (130°C).
- Step 3: Unreacted and excess ammonia (in the vaporized state) is collected from the top of each tank and sent to the source container of the liquid ammonia to reduce excess loss.
- Step 4: The hot slurry, containing about 16 to 20% water, is then pumped into the granulator.
- Step 5: More ammonia is added to the granulator to increase the molar ratio to approximately 2.0.
Granulation
- Step 6: From the third neutralizer, the hot slurry is mixed with KCl.
- Step 7: Then the KCl mixed slurry is absorbed in a bed of dry recycled fertilizer, which is moving through a rotating drum granulator.
- Step 8: A rotary adiabatic dryer then reduces the moisture to less than 1%.
- Step 9: The resulting Dried product is then separated into three parts on a double-deck screen.
- Step 10: Thereafter, a small section of the product from the lower screen of the deck is transferred for the bagging operations.
- Step 11: The oversized and the smaller-sized granules are again sent to the granulator for further processing.
Powder ammonium phosphate
Ammonium phosphate is powdered in used as a fertiliser for its high phosphorus content (as P2O5).
Besides this, a few fertilizers, such as ammonium phosphate sulphate fertilizer, ammonium phosphate-chloride, and ammonium phosphate-nitrate, are produced by different types of processes, which include the neutralization of ammonia with a mixture of phosphoric acid. A few plant waste acids like sulfuric acid, nitric acid, or hydrochloric are also added. The fertilizers thus formed are free-flowing and less hygroscopic as compared to the individual components. These fertilizers are also successfully used in different types of soil.
Also read :
Uses of Ammonium phosphate (Mono Ammonium Phosphate Uses)
- These fertilizers are used as highly effective and non-chloride nitrogen, potassium-containing compound fertilizers in agriculture. Its nitrogen and phosphorus content is totally (N+P2O5) about 73%.
- This is also used in manufacturing yeast, vinegar, yeast foods, and bread improvers.
- This can be used in buffer solutions and in an analytical chemistry laboratory
- This can also be applied in preventing the fabric from fire, fire prevention coating, and as a dry powder for a fire extinguisher.
- It is used as a fermenting agent, a nourishing agent in food-grade products.

Also check-
Some Solved Examples
Question 1: What is the formula of ammonium phosphate?
A) $\mathrm{NH}_4 \mathrm{PO}_4$
B) $\left(\mathrm{NH}_4\right)_3 \mathrm{PO}_4$
C) $\left(\mathrm{NH}_4\right)_2 \mathrm{PO}_4$
D) $\mathrm{NH}_3 \mathrm{PO}_4$
Solution:
Ammonium phosphate is a chemical compound composed of ammonium ions $\mathrm{NH}_4^{+}$and phosphate ions $\mathrm{PO}_4^{3-}$. The correct formula for ammonium phosphate is $\left(\mathrm{NH}_4\right)_3 \mathrm{PO}_4$.
Hence, the correct answer is option (2)
Question 2: Ammonium phosphate is primarily used as a:
A) Fuel
B) Fertilizer
C) Catalyst
D) Solvent
Solution: Ammonium phosphate is widely used as a fertilizer due to its high nitrogen and phosphorus content, which are essential nutrients for plant growth. It helps improve soil fertility and promotes healthy plant development.
Hence, the correct answer is option (2)
Question 3: Ammonium phosphate can be formed by the reaction of ammonia $\left(\mathrm{NH}_3\right)$ with:
A) Phosphoric acid $\left(\mathrm{H}_3 \mathrm{PO}_4\right)$
B) Sulfuric acid $\left(\mathrm{H}_2 \mathrm{SO}_4\right)$
C) Nitric acid $\left(\mathrm{HNO}_3\right)$
D) Hydrochloric acid $(\mathrm{HCl})$
Solution:
Ammonium phosphate is typically formed by reacting ammonia $\left(\mathrm{NH}_3\right)$ with phosphoric acid $\left(\mathrm{H}_3 \mathrm{PO}_4\right)$. The reaction forms ammonium phosphate as follows:
$3 \mathrm{NH}_3+\mathrm{H}_3 \mathrm{PO}_4 \rightarrow\left(\mathrm{NH}_4\right)_3 \mathrm{PO}_4$
Hence, the correct answer is option (1)
Question 4: Which of the following ions are present in the structure of ammonium phosphate?
A) Ammonium ions and sulfate ions
B) Ammonium ions and phosphate ions
C) Sodium ions and phosphate ions
D) Potassium ions and sulfate ions
Solution:
Ammonium phosphate consists of ammonium ions $\left(\mathrm{NH}_4{ }^{+}\right)$and phosphate ions $\left(\mathrm{PO}_4{ }^{3-}\right)$. These ions combine to form the compound ammonium phosphate, which is used as a fertilizer.
Hence, the correct answer is option (2)
Frequently Asked Questions (FAQs)
149gm (molecular weight) of (NH4)3PO4 contains 12g of H.
3.18 moles of H = 3.18g of H
Therefore, 3.18gm of H present in (149×3.18)/ 12= 39.5gm (NH4)3PO4.
Now, 149gm of (NH4)3PO4 contains 64g of Oxygen.
Therefore, 39.5gm of (NH4)3PO4 will contain = (64×39.5)/ 149
=16.96 gm oxygen
Hence, 16.96g oxygen present in the sample.
Therefore, No. of moles of oxygen=16.96/ 16= 1.06 moles
$4\left(\mathrm{NH}_4\right)_3 \mathrm{PO}_4+3 \mathrm{~Pb}\left(\mathrm{NO}_3\right)_4 \rightarrow \mathrm{~Pb}_3\left(\mathrm{PO}_4\right)_4+12 \mathrm{NH}_4 \mathrm{NO}_3$
Ammonium sulfate (NH4)2SO4 on heating gives ammonium bisulfate initially. On further heating ammonium bisulphates decomposes into NH3, N, SO2 and H2O.
(NH4)3PO4
115.03gm/mole
