Electromeric effect
The instantaneous formation of a dipole in the molecule of an organic compound due to the complete transfer of shared pi electron pairs to one of the atoms under the influence of an attacking reagent is referred to as the Electromeric effect. This effect can be observed in organic compounds that contain at least one multiple bond. When the atoms participating in this multiple bonds come under the influence of an attacking reagent, one pi-bonding pair of electrons is completely transferred to one of the two atoms. The electromeric effect is a temporary effect that remains as long as the attacking reagent is present and exposed to the organic compound.
This Story also Contains
- Electromeric Effect
- Solved Examples Based on Electromeric Effect
- Conclusion
In this article, we will cover the topic (Hyperconjugation). This topic falls under the broader category of (Some Basic Principles of Organic Chemistry), which is a crucial chapter in (Class 11 Chemistry).
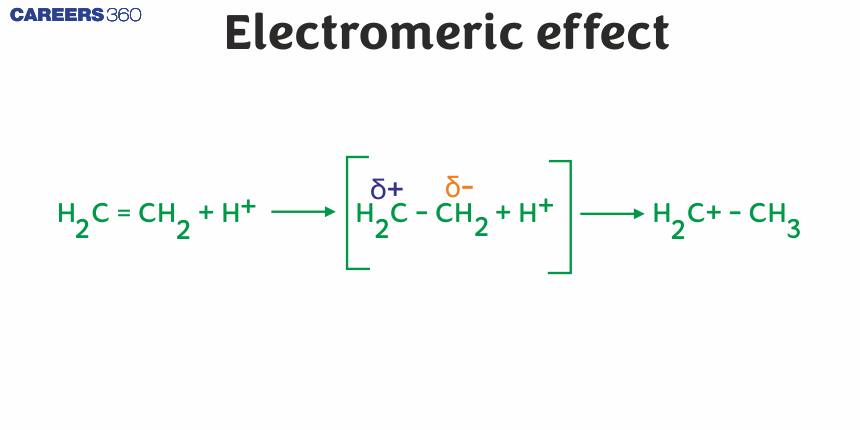
Electromeric Effect
It is a temporary effect. The organic compounds having a multiple bond (a double or triple bond) show this effect in the presence of an attacking reagent only. It is defined as the complete transfer of a shared pair of π-electrons to one of the atoms joined by a multiple bond on the demand of an attacking reagent.
The effect is annulled as soon as the attacking reagent is removed from the domain of the reaction. It is represented by E.
In this effect the π−electrons of the multiple bond are transferred to that atom to which the reagent gets attached. For example:
This phenomenon occurs when the pi bond’s electron pair is shifted toward the attacking reagent. The +E effect is visible when acid is added to alkenes. A positive electron transfer (or +E effect) occurs when an electrophile attacks a positively charged atom and the pi electrons are transferred to the positively charged atom. The protonation of ethene is an illustration of the +E effect. This phenomenon occurs when the attacking reagent’s electron pair is shifted away from the pi bond’s electron pair. The attacking reagent binds to the molecule’s positively charged atom, i.e., the atom that lost an electron pair during the transfer.
Recommended topic video on(Electromeric effect)
Solved Examples Based on Electromeric Effect
Q.1 Displacement of $\pi$ electron of a multiple bond towards the atom or away from the atom at the demand of reagent is called
(1) Electromeric effect
(2) Inductive effect
(3) Mesomeric effect
(4) Hyperconjugation
Solution:
As we have learned
The displacement of $\pi$ electrons in multiple bonds towards the atom or away from the atom at the demand of a reagent is called the electromeric effect.
Hence, the answer is the option (1).
Q.2 Select an incorrect statement about Electromeric effect-
(1) It involves polarisation of $\pi$ electrons in the presence of a reagent
(2) It is a temporary effect
(3) Electrophilic reagents are generally the cause for this effect in Alkenes
(4) The polarisation of $\pi$ electrons persist even after the reagent has been removed from the system
Solution:
As we have learned
The electromagnetic effect is a temporary effect in which the $\pi$ electrons are polarised in the presence of an attacking reagent.
The polarization is not present in the absence of the reagent
Hence, the answer is the option(4).
Q.3 The interaction between the π bond and a lone pair of electrons present on an adjacent atom is responsible for :
1) (correct)Resonance effect
2)Electromeric effect
3)Inductive effect
4)Hyperconjugation
Solution
→ The interaction between the π bond and the lone pair of electrons present on the adjacent atom is responsible for the resonance effect.
$\overbrace{\mathrm{CH}_2}=\mathrm{CH}-\stackrel{\ominus}{\mathrm{O}} \mathrm{H} \longleftrightarrow \stackrel{\ominus}{\mathrm{C}} \mathrm{H}_2-\mathrm{CH}=\stackrel{\oplus}{\mathrm{O}} \mathrm{H}$
Conclusion
Inductive, electromeric, and resonance effects are three of the most frequently observed electronic effects in an organic reaction generated by the attacking reagent. Each of these three effects results in the formation of polarity in the organic substrate. The electromeric effect can be defined as a transient effect that creates polarity in an organic molecule with pi-linked atoms. Electromeric interactions are categorized as$+E$ or $-E$ depending on the atom with which the assaulting reagent engages.
