Electron Affinity - Introduction, Definition, Trends, FAQs
Some atoms eagerly receive electrons while others strongly resist them. What determines the readiness of an atom to gain an electron and form a stable negative ion? Electron affinity explains this behavior by describing the energy change accompanying the addition of an electron to an isolated gaseous atom, which helps understand periodic trends and explains the chemical reactivity of elements.
This Story also Contains
- Electron Affinity (EA)
- First and Second Electron Affinity
- Electron Affinity of Halogens
- Electron Affinity Trend
- Important Exceptions
- Some Solved Examples
Electron Affinity (EA)
Electron affinity is the energy released when an isolated gaseous atom gains an electron to form a negative ion. In other words, the probability of a neutral atom gaining an electron.
- Electron affinity is usually expressed in $\mathrm{kJ} \mathrm{mol}^{-1}$.
- More negative value → higher electron affinity.
- It is a periodic property and depends on atomic size and nuclear charge.
$\mathrm{X}(\mathrm{g})+\mathrm{e}^{-} \rightarrow \mathrm{X}^{-}(\mathrm{g})+$ Energy
First and Second Electron Affinity
-
First EA: Energy released when first electron is added (usually exothermic).
-
Second EA: Energy required to add an electron to a negatively charged ion (always endothermic).
Read more :
- NCERT notes Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 3 Classification of Elements and Periodicity in Properties
Electron Affinity of Halogens
On the periodic table, the halogens are to the left of the noble gases. Fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (A) are the five toxic non-metallic elements that make up Group 17 of the periodic table (At). Even though astatine is radioactive and has only short-lived isotopes, it behaves similarly to iodine and is commonly categorized as a halogen. Because halogen elements have seven valence electrons, forming a full octet requires only one additional electron. Because of this, they are more reactive than other non-metal groups.
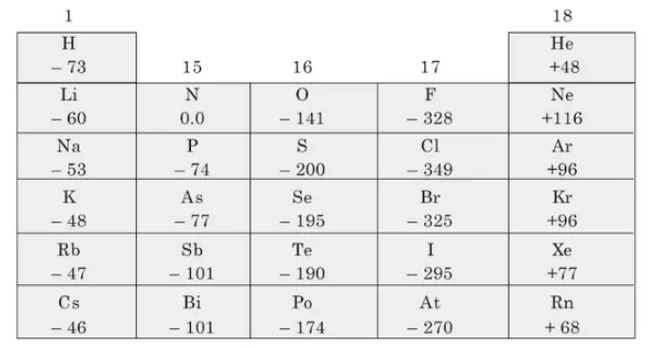
Electron Affinity (decreases down the group) As atomic size decreases electron affinity decreases $(\mathrm{At}<\mathrm{l}<\mathrm{Br}<\mathrm{F}<\mathrm{Cl})$. The nucleus will be less attractive to an electron, resulting in a low electron affinity. Fluorine, on the other hand, has a lower electron affinity than chlorine. This is due to fluorine's smaller size when compared to chlorine.
Electron Affinity Trend
-
The greater the distance between two objects, the less attraction there is; thus, when an electron is added to the outside orbital, less energy is released. Furthermore, an element with more valence electrons is more likely to gain electrons and form a stable octet. An atom with very few valence electrons is less likely to gain electrons.
-
However, because the number of valence electrons increases as the group number decreases, one might assume that the element will be more stable and have a higher electron affinity. The shielding effect is not taken into account. As the period decreases, the shielding effect increases, causing electrons to repel one another. This is why, as one moves down the periodic table, the attraction between the electron and the nucleus decreases.
-
The first electron affinities become less as you move down the group (in the sense that less energy is evolved when the negative ions are formed). Fluorine deviates from this pattern and must be accounted for separately. The strength of the attraction between the incoming electron and the nucleus is measured by the electron affinity; the stronger the attraction, the more energy is released. Nuclear charge, distance, and screening are the same factors that influence this attraction as they are for ionization energies. Extra screening electrons offset the increased nuclear charge as you move down the group.
Important Exceptions
-
Halogens have the highest electron affinity.
-
Chlorine > Fluorine
-
Due to small size of F, electron–electron repulsion is high.
-
-
Noble gases have positive or zero EA (stable configuration).
-
Group 2 and Group 15 elements have low or near-zero EA due to stable subshells.
Also check-
Some Solved Examples
Question 1: Choose the correct order of electron gain enthalpy or electron affinity among the following:
1) O > F
2) (correct) S > O
3) N > O
4) N > F
Solution :
The electron affinity of elements increases from left to right in a period and decreases in moving down the group.
Hence, the answer is option (2).
Question 2: The correct group of isoelectronic species
1) $\mathrm{Al}^{3+}, \mathrm{Si}^{2+}, \mathrm{Na}^{+}$
2) $\mathrm{Cl}^{-}, \mathrm{F}^{-}, \mathrm{Br}^{-}$
3) $\mathrm{H}^{+} \mathrm{Li}^{+} \mathrm{Na}^{+}$
4) (correct) $\mathrm{Na}^{+}, \mathrm{Mg}^{2+}, \mathrm{Al}^{3+}$
Solution :
As we learn
Isoelectronic species -
A series of atom, ions, and molecules in which each species contains the same number of electrons but different nuclear charge.
- wherein
e.g. $\mathrm{N}^{3-}, \mathrm{O}^{2-}, \mathrm{F}^{-}, \mathrm{Ne}, \mathrm{Na}^{+}, \mathrm{Mg}^{2+}, \mathrm{Al}^{3+}$
$\mathrm{Na}^{+}, \mathrm{Mg}^{2+}, \mathrm{Al}^{3+}$ all have the same number of electrons 10 , but they have a different number of nuclear charge
Hence, the answer is option (4).
Question 3: Which among the following factors is the most important in making fluorine the strongest oxidising agent?
1) Electron affinity
2) Ionization energy
3) (correct) Hydration enthalpy
4) Bond dissociation energy
Solution:
There are two factors that affect fluorine's oxidizing power, namely Hydration enthalpy and Bond energy. But of them, Hydration enthalpy plays a greater role than the other.
Hence, the answer is option (3).
Question 4: The order of increasing electron affinity of the electronic configurations is
1) $1 s^2 2 s^2 2 p^6 3 s^2 3 p^5$
2) $1 s^2 2 s^2 2 p^3$
3) $1 s^2 2 s^2 2 p^5$
4) $1 s^2 2 s^2 2 p^6 3 s^1$
1) (correct) $2<4<3<1$
2) $1<2<3<4$
3) $1<3<2<4$
4) $4<3<2<1$
Solution:
Configurations 1 and 3 refer to the halogens Cl and F, respectively. They have a high value of electron affinity.
Configuration 2 refers to a stable, half-filled electronic configuration that has no tendency to accept an additional electron.
Configuration 4 refers to alkali metals, which are electropositive and have less tendency to accept electrons.
From above
half-filled electronic configuration < alkali metals < halogens
order of electron gain enthalpy
-349 (CI) <-328 (F) < alkali < half filled
order of electron affinity (reverse of EGE)
-349 $(\mathrm{CI})>-328(\mathrm{~F})>$ alkali > half-filled
The order will be
$2<4<3<1$
Hence, the answer is option (1).
Practice more questions with the link given below
Frequently Asked Questions (FAQs)
Because the incoming electron will be closer to the nucleus in fluorine than in any other of these elements, a high value of electron affinity is expected. However, because fluorine is such a small atom, the new electron is inserted into a region of space that is already densely populated with electrons, causing significant repulsion. This repulsion reduces the incoming electron's attraction, lowering the electron affinity. In Group 16, there is a similar reversal of the expected trend between oxygen and sulfur. For the same reason that fluorine's first electron affinity is smaller than chlorine's, oxygen's (-142 kJ mol-1) is smaller than sulfur’s (-200 kJ mol-1).
Metals have a low electron affinity (a lower probability of gaining electrons) because they prefer to give up their valence electrons rather than gain an electron, which requires more energy. Furthermore, because they are far from the nucleus, they do not exert a strong pull on the valance electrons, and thus have less energy to attract them.
Because non-metals have more valence electrons than metals, it is easier for them to gain electrons rather than lose valance electrons to complete a stable octet. Furthermore, the valance electrons of non-metals are closer to the nucleus, allowing for greater attraction between the two.
Electron affinity decreases as you progress down the periodic table. The atom does not want to gain electrons because there is the minimum charge on the outer energy levels from the nucleus; second, the shielding effect increases, causing repulsion between the electrons, causing them to move further away from each other; and finally, the shielding effect increases, causing repulsion between the electrons, causing them to move further away from each other.
Some of the key differences between electronegativity and electron affinity are listed below.
Electronegativity is the property that causes the electron to be drawn to the atom. Electron affinity, on the other hand, is concerned with the energy released when an electron is added to an atom.
Electronegativity is a qualitative characteristic, whereas electron affinity is a quantitative characteristic.
Although electronegativity is a unitless quantity, it is defined in terms of Pauling. In contrast, electron affinity is measured in kJ/mol.
The electronegativity of an element is higher when that element has a stronger attracting ability. If the element's nuclear charge is higher, however, electron affinity is higher.
Electronegativity is usually measured in the range of 0.7 to 3.98. Electron affinity, on the other hand, is known to be fixed because when an electron is added to an atom, it releases nearly the same amount of energy.
Fluorine is the most electronegative element known, while chlorine has the highest electron affinity.
