Electronic Configuration of First 30 Elements - Meaning, Definition, Elements, Functions, FAQs
Have you ever thought about why sodium is highly reactive while neon is completely inert? Or why do elements in the same group show similar chemical properties? The answer lies in the electronic configuration, the systematic distribution of electrons into different shells, subshells, and orbitals. Electronic configuration is an organised alignment of electrons in atomic orbitals according to the energy levels. This arrangement of electrons is directed by concepts like Hund's rule, Pauli exclusion, and the Aufbau rule.
This Story also Contains
- Electronic Configuration
- How To Write The Electronic Configuration Of Elements
- Rules for Writing Electronic Configurations Of Elements
- List Of First 30 Elements: Atomic Number, Element, And Electronic Configuration
- Function of Electronic Configuration Of Elements
- Some Solved Examples

Electronic configuration helps determine the chemical properties, reactivity, and position in the periodic table. It also influences what type of bond the elements will form, the valency, and the ability to donate or accept electrons during a chemical reaction. The trend in the periodic table, like ionisation enthalpy, electronegativity, and atomic size, can also be explained through this arrangement of electrons. The following electronic configurations of the first 30 elements are mentioned, which will help in understanding the trends they obey.
Electronic Configuration
An electronic Configuration, also known as an electronic structure, is the arrangement of electrons at different energy levels surrounding an atomic nucleus. The electronic configuration of a molecule is the distribution of electrons in distinct molecular orbitals. The importance of the molecule cannot be overstated. It is possible to determine the number of electrons in bonding and antibonding molecular orbitals from a molecule's or molecular ion's electronic configuration.
-
The electrical configuration of an element is used to figure out where electrons are located in that element.
-
From the lowest to the highest energy level, electrons are arranged in ascending order.
-
The electrical configuration of an element is largely determined by its atomic number.
-
The electrical configuration of an atom helps determine an element's valency, which aids in determining the element's reactivity.
-
The atomic spectra can also be interpreted using the electronic configuration.
-
Noble gases with totally filled outermost electrons, such as Neon, Argon, and Helium, are the most stable. Noble gases have filled valence shells, which give them their inertness.
-
Copper and chromium have a peculiar electrical structure in which the 3d orbitals are filled first, rather than the 4s orbitals.
-
In chromium $\left([\mathrm{Ar}] 3 \mathrm{~d}^5 4 \mathrm{~s}^1\right)$, the d-orbital, which is filled with single electrons, boosts the atom's stability. Similarly, the d-orbital of Copper $[\mathrm{Ar}] 3 \mathrm{~d}^{10} 4 \mathrm{~s}^1$ is totally filled with paired electrons, ensuring the atomic structure's stability.
How To Write The Electronic Configuration Of Elements
The rules mentioned below are to be followed while writing electronic configurations. To get different quantum numbers, we first have to extract various information, such as the number of electrons, the possible number of various shells and orbitals, energy levels, etc., of elements by using the periodic table.
The electronic configuration is as follows: $1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 4 s^1$. Its 19 electrons can be divided into shells in the following ways:
K shell (n=1) equals 2, L shell (n=2) equals 8, M shell (n=3) equals 8, and N shell (n=4) equals 1.
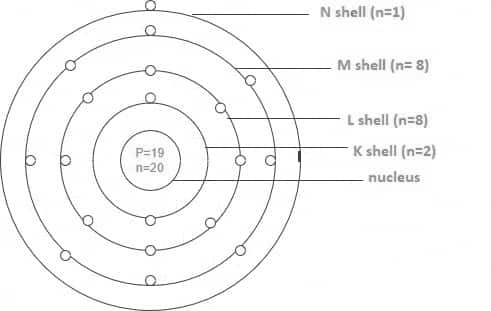
Let us take an example of the electronic configuration of iron, which is mentioned below, for a better understanding of the topic
Iron is a one-of-a-kind element that exists both outside and inside us. The atomic number of Iron is 26. Iron has 8 valence electrons and an electron configuration of $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^6 4 s^2$, which means it has
-
K shell – 2 electrons,
-
L shell – 8 electrons,
-
M shell – 14 electrons, and
- N shell – 2 electrons.
Various properties of Iron can be explained using the electronic configuration. Like, Iron is a silvery white metal that is ductile and malleable under normal conditions. Iron is a medium-activity metal that extracts hydrogen from water solutions of strong acids like HCl and sulfuric acid, resulting in the formation of iron salts. These can be explained by the number of valence shell electrons in Iron.
Rules for Writing Electronic Configurations Of Elements
-
The Aufbau principle states that before occupying an orbital associated with a higher energy level, electrons must entirely fill the atomic orbitals of the previous energy level. In the sequence of increasing orbital energy levels, electrons occupy orbitals of lower energy first.
-
According to Pauli's exclusion principle, no two electrons may have the same values for all four quantum numbers. As a result, each subshell of an orbital may only hold a maximum of two electrons, both of which must have opposite spins for the spin quantum number to be different.
-
Hund's rule of maximum multiplicity states that all subshells in an orbital must be occupied singly before any subshell can be twice occupied. Furthermore, all electrons in singly occupied subshells must have the same spin (in order to maximise the overall spin).
-
Also Read:
List Of First 30 Elements: Atomic Number, Element, And Electronic Configuration
|
ATOMIC NUMBER 1 to 30 |
ELEMENT |
ELECTRONIC CONFIGURATION |
|
1 |
HYDROGEN (H) | $1 \mathrm{~s}^1$ |
|
2 |
HELIUM (He) | $1 \mathrm{~s}^2$ |
|
3 |
LITHIUM (Li) | $1 s^2 2 s^1$ |
|
4 |
BERYLLIUM (Be) | $1 s^2 2 s^2$ |
|
5 |
BORON (B) | $1 s^2 2 s^1 2 p^1$ |
|
6 |
CARBON (C) | $1 s^2 2 s^1 2 p^2$ |
|
7 |
NITROGEN (N) | $1 s^2 2 s^1 2 p^3$ |
|
8 |
OXYGEN (O) | $1 s^2 2 s^1 2 p^4$ |
|
9 |
FLUORINE (F) | $1 s^2 2 s^1 2 p^5$ |
|
10 |
NEON (Ne) | $1 s^2 2 s^1 2 p^6$ |
|
11 |
SODIUM (Na) | $1 s^2 2 s^1 2 p^6 3 s^1$ |
|
12 |
MAGNESIUM (Mg) | $1 s^2 2 s^1 2 p^6 3 s^2$ |
|
13 |
ALUMINIUM (Al) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^1$ |
|
14 |
SILICON (Si) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^2$ |
|
15 |
PHOSPHORUS (P) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^3$ |
|
16 |
SULPHUR (S) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^4$ |
|
17 |
CHLORINE (Cl) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^5$ |
|
18 |
ARGON (Ar) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6$ |
|
19 |
POTASSIUM (K) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 4 s^1$ |
|
20 |
CALCIUM (Ca) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 4 s^2$ |
|
21 |
SCANDIUM (Sc) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^1 4 s^2$ |
|
22 |
TITANIUM (Ti) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^2 4 s^2$ |
|
23 |
VANADIUM (V) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^3 4 s^2$ |
|
24 |
CHROMIUM (Cr) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^5 4 s^1$ |
|
25 |
MANGANESE (Mn) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^5 4 s^2$ |
|
26 |
IRON (Fe) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^6 4 s^2$ |
|
27 |
COBALT (Co) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^7 4 s^2$ |
|
28 |
NICKEL (Ni) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^8 4 s^2$ |
|
29 |
COPPER (Cu) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^{10} 4 s^1$ |
|
30 |
ZINC (Zn) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^{10} 4 s^2$ |
The above table contains the atomic numbers 1 to 30, elements with symbols and electronic configuration. The electronic configuration of elements can also be written in the form of the electronic configuration of the nearest noble gases.
For example, in this table, we can represent the electronic configuration of elements from 21 to 30 in the form of Ar as Argon (atomic number - 18) is the nearest noble gas for the first series elements of the d-block.
Function of Electronic Configuration Of Elements
The chemical characteristics of elements are largely determined by their electronic arrangement. Despite their small size, electrons are responsible for determining the nature of the elements. They determine the valency, ionisation potential, ionisation enthalpy, chemical bonding, and practically all other chemical properties of the element. When an element lacks an electron, it is classified as an electron acceptor, and when it has an excess electron, it is classified as an electron donor. As a result, electrical configuration, like the electron, is a deciding factor.
Also read -
Some Solved Examples
Question 1: Which law indicates the pairing of electrons in the same orbital?
1) Newton’s first law
2) (correct) Hund’s rule
3) Aufbau principle
4) Pauli exclusion principle
Solution
Hund’s rule states that “pairing of electrons in the orbitals belonging to the same subshell (p, d or f) does not take place until each orbital belonging to that subshell has got one electron each. It is singly occupied”.
Hence, the answer is option (2).
Question 2: The number of electrons that Vanadium (Z = 23) has in p-orbitals is equal to ______
1) (correct) 12
2) 11
3) 10
4) 9
Solution
The electronic configuration of V(Z= 23) is given as
$1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 4 s^2 3 d^3$
Thus, there are 12 electrons in the p-orbitals of Vanadium.
Hence, the answer is option (1).
Question 3: Identify the element for which the electronic configuration in the +3 oxidation state is [ Ar ] 3d5:
1) Ru
2) Mn
3) Co
4) Fe
Solution
As we have learned,
Fe has an electronic configuration of [Ar] 4s23d6
So, Fe3+ has an electronic configuration [Ar] 3d5.
Hence, the answer is option (4).
Question 4: Which of the following configurations represents a noble gas?
A. $1 s^2 2 s^2 2 p^6$
B. $1 s^2 2 s^2 2 p^6 3 s^2 3 p^5$
C. $1 s^2 2 s^2 2 p^6 3 s^2 3 p^6$
D. $1 s^2 2 s^2 2 p^3$
Solution- $1 s^2 2 s^2 2 p^6 3 s^2 3 p^6$ totals 18 electrons = Argon (Ar), a noble gas.
Noble gases have full outer shells, making them stable.
Hence, the correct answer is option (C)
Question 5: The correct electronic configuration of Cr (Atomic No. 24) is:
A. $[A r] 3 d^4 4 s^2$
B. $[A r] 3 d^5 4 s^1$
C. $[A r] 3 d^3 4 s^3$
D. $[A r] 3 d^6$
Solution:
Cr attains extra stability due to half-filled $\left(3 \mathrm{~d}^5\right)$ configuration: $[A r] 3 d^5 4 s^1$
Hence, the correct answer is option (B)
Practice More Questions With The Link Given Below
Frequently Asked Questions (FAQs)
Noble gas configurations are particularly stable due to a full valence shell of electrons. Elements tend to gain, lose, or share electrons to achieve a noble gas configuration, which is a driving force behind chemical reactions.
In nitrogen (N), which has the electronic configuration 1s² 2s² 2p³, the three electrons in the 2p subshell will occupy separate p orbitals before any pairing occurs. This arrangement minimizes repulsion and stabilizes the atom.
Orbitals are regions in an atom where electrons are likely to be found. They come in different shapes (s, p, d, f) and energy levels, and they dictate how electrons are arranged in an atom's electronic configuration.
Transition metals have partially filled d orbitals. Their electronic configurations can exhibit irregularities due to the stability associated with half-filled and fully filled subshells, leading to variations in expected configurations.
As you move across a period in the periodic table, the atomic number increases, leading to an increase in the number of electrons. This results in a gradual filling of orbitals, following the rules of electron configuration.
Atomic mass is the weighted average mass of an element's isotopes, measured in atomic mass units (amu). It accounts for both the mass of protons, neutrons, and the relative abundance of each isotope.
