Glucose - Meaning, Definition, History, Properties, FAQs
What is Glucose?
Glucose Definition: Glucose formula is C6H12O6 chemical name is glucose. One of the most abundant monosaccharides, glucose belongs to the carbohydrate subcategory. The most abundant carbohydrate in the world, glucose is made by plants and most algae using sunlight to make it from water and carbon dioxide, which is used to make cellulose in their cell walls.
All organisms derive most of their energy from glucose in the process of metabolism. In plants, glucose is stored as starch and amylopectin, while in animals it is stored as glycogen. Animals have blood sugar that is made up of glucose.
It is the naturally occurring form of glucose, d-glucose, which is of lesser importance than the synthetic form l-glucose. There are six carbon atoms in glucose and therefore it is an aldohexose, not a monosaccharide. There are two types of glucose molecules: open-chain (acyclic) and ring (cyclic). Fruits and other parts of plants naturally contain glucose, and most plants store it in their free state. Glycogenolysis occurs in animals when glycogen is broken down. Among the safest and most effective medicines needed in a health system, glucose is a member of the World Health Organization's List of Essential Medicines. Combining it with sodium chloride also makes the list.
Also read -
History of Glucose
The German chemist Andreas Margraves isolated glucose from raisins for the first time in 1747. The distinction between grape sugar and cane sugar (sucrose) was made by Johann Tobias Levitz in 1792. Chemical literature has used the term glucose, which was coined in 1838 by Jean Baptiste Dumas. Aqueous solutions of glucose are polarized in the right direction in an aqueous solution of dextrose (from Latin dexter = right). In contrast, linearly polarized light is turned to the right by d-fructose (a ketohexose) and l-glucose. Chemical name of glucose is D-glucose.
A correct understanding of glucose provided a significant advance in the understanding of organic chemistry since glucose is a basic necessity for many organisms. As a result of Emil Fischer's investigations, who received the 1902 Nobel Prize in Chemistry for his findings, these notions were understood largely. Using Can't Hoff's theory of asymmetrical carbon atoms, Fischer correctly predicted all the possible isomers of sugars between 1891 and 1894. These compounds were originally used to name natural substances. Due to systematic nomenclatures, which take into account absolute stereochemistry (such as the Fischer nomenclature and D/L nomenclature), the two enantiomers have been given the same name.
Related Topics Link |
Chemical properties
Water and acetic acid display high solubility for glucose solids, but methanol and ethanol exhibit poor solubility. Hexose, with its six carbons, is one of the monosaccharides, a subgroup of sugars. Among the sixteen stereoisomers of aldohexose, d-glucose is one. Nature has both d-glucose and l-glucose, but the l-isomer, l-glucose, does not occur. The chemical process of hydrolysis is used to obtain glucose from carbohydrates like milk sugar (lactose), cane sugar (sucrose), maltose, cellulose, glycogen, etc.
Commercially, dextrose is manufactured from corn starch in the US and Japan, from potato and wheat starch in Europe, and from tapioca starch in tropical areas. During the manufacturing process, pressurized steam is used in a jet that hydrolyses the material at a controlled pH, followed by further enzymatic depolymerization. Honey is primarily composed of unbonded glucose. The glucose molecules are colourless and easily soluble in water, acetic acid, and several other solvents. Methanol and ethanol are only sparingly soluble in these compounds.
Also read :
An overview of the nomenclature and structure
A monohydrate of glucose with a closed pyran ring (dextrose hydrate) is usually present in solid form. It is present in aqueous solution as an open-chain to a small extent which interconverts primarily (see mutation). Crystallization of the three known forms: 5-glucopyranose, 5-glucopyranose, and 5-glucopyranose hydrate is possible from aqueous solutions. A building block for complex polysaccharides such as starch, amylopectin, glycogen, and cellulose, glucose is a disaccharide found in lactose and sucrose (cane or beet sugar). It has been determined that glucose has a glass transition temperature of 31 °C and the Gordon-Taylor constant (a constant set up through experiment to predict a mixture's glass transition temperature for different mass fractions of two substances) is 0.92.
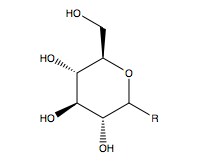
NCERT Chemistry Notes:
Glucose
Properties of Glucose
C6H12O6 | Glucose |
Molecular Weight/ Molar Mass | 180.16 g/mol |
Density | 1.54 g/cm³ |
Simple sugar | Monosaccharide |
Also check-
Questions related to
On Question asked by student community
Correct Answer: pyruvate
Solution : The correct option is pyruvate.
Glycolysis is the process of breaking glucose molecules to generate energy. In this process, the glucose is broken down to create pyruvate in a cell's cytoplasm. The breakdown of one glucose molecule into two pyruvate molecules results in the
Correct Answer: Hydrogen peroxide
Solution : The correct option is Hydrogen peroxide.
In the paper industry, hydrogen peroxide (H2O2) is often used for pulp bleaching. It is a non-toxic bleaching chemical that aids in the removal of colour, and residual lignin from the wood pulp during
