Structure of Glucose and Fructose - Properties, Types, Steps with FAQs
Have you ever thought why glucose tastes sweet yet serves as the primary energy source in our body, while fructose, equally sweet, is called 'fruit sugar”? How do two compounds with the same molecular formula $\left(\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6\right)$ show different properties just because of a difference in structure? We will get these answers by understanding the structure of glucose and fructose. Glucose is an aldohexose (it contains an aldehyde group), whereas fructose is a ketohexose (it contains a ketone group).
This Story also Contains
- Glucose
- Properties of Glucose
- Glucose and Fructose Formula
- Structure of Glucose and Fructose
- Fructose Structural Formula
- Cyclic Structure Of Glucose
- Pyranose Structure Of Glucose
- Configuration Of Glucose
- Preparation Of Glucose From Starch
- Test of Glucose
- Some Solved Examples
Glucose is an Aldo-hexose (monosaccharide with 6 C atoms and an aldehyde group) with a chemical formula C6H12O6. The natural form of glucose, i.e., D-glucose, is also known as dextrose. It can exist in a linear form or a pyranose form (5 C atoms with an O atom in the ring). Glucose is an aldohexose monosaccharide with a ubiquitous nature that acts as the main substrate in glycolysis in living tissues. In this article, we under the concept of glucose and fructose, which is covered in the Biomolecules of Class 12. This concept is important for board exams and Joint Entrance Examination (JEE Main) and National Eligibility Entrance Test (NEET), and other such entrance exams.
Glucose
Glucose is a monosaccharide present in living cells that is used as a source of energy. Glucose is the main product of photosynthesis and is utilized during cellular respiration in both prokaryotic and eukaryotic organisms.
Properties of Glucose
|
C6H12O6 |
Glucose |
|
Molecular Weight/ Molar Mass |
180.16 g/mol |
|
Density |
1.54 g/cm³ |
|
Melting Point |
146 °C |
|
Simple sugar |
Monosaccharide |
|
Glucose boiling point |
527.1±50.0 °C at 760 mmHg |
Glucose and Fructose Formula
The chemical formula or structural formula of glucose is C6H12O6. The chemical formula or structural formula of fructose is also C6H12O6. Though glucose and fructose have similar chemical formulas, they differ from each other structurally and stereochemically. And causes differences in molecules despite of sharing the same atoms in the same proportion. Better to say, they are isomers of each other or isomeric monosaccharides.
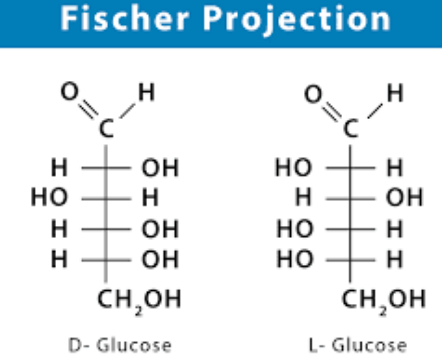
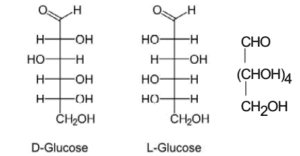
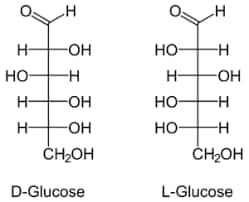
D-Glucose Formula
D-glucose basically means dextrorotatory (meaning that as an optical isomer, when placed inside a polarimeter, that rotates the plane of polarized light to the right and also an origin for the D designation) glucose. This is often termed as dextrose. it is one of the stereoisomers of glucose and is the one that is biologically active. It occurs in plants as a major product of photosynthesis. In animals and fungi, it is the result of the breakdown of glycogen via glycolysis.
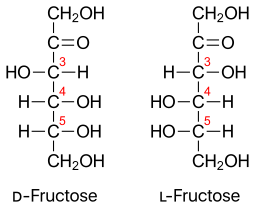
Structure of Glucose and Fructose
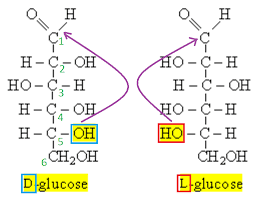
D and L Fructose
Related Topics Link,
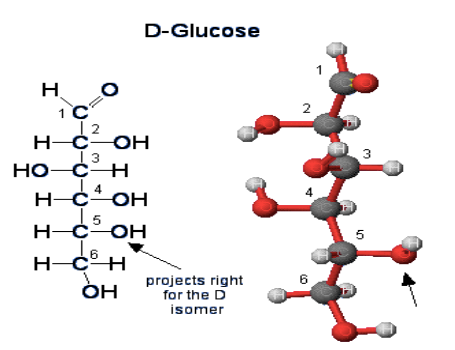
Steps To Draw The Open Chain Structure Of A Glucose Molecule
Step 1: 6 carbon atoms are drawn at first.
Step 2: the arms for all the carbon atoms excluding the first one are extended.
Step 3: thereafter, hydrogen-to-carbon bonds are drawn such that four are on one side and the remaining one on the other side.
Step 4: The remaining spaces should be filled with a hydroxyl group. (Important – transpose
(OH) to —> (HO) for the left side to show that the oxygen is bonded to carbon)
Step 5: The ends of the chain should be completed with two single-bonded hydrogen bonds and one double-bonded carbon.
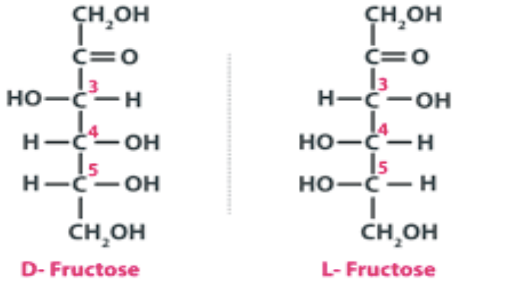
Fructose Structural Formula
The chemical or structural formula of Fructose is as same as that of glucose, i.e., C6H12O6. But they are stereogenically different from each other hence, isomers to each other. Open chain structure of fructose (fructose linear structure). Fructose is a polyhydroxyketone having 6 carbon atoms within it.
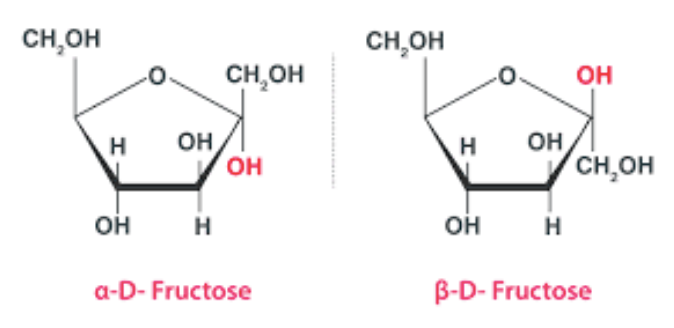
Cyclic Structure of Fructose
Crystalline fructose implements a cyclic 6-membered structure, termed β-D-fructopyranose. This is to stabilize its hemiketal and internal hydrogen bonding within it.
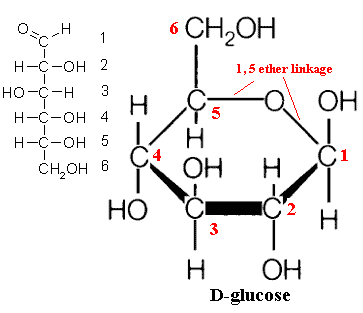
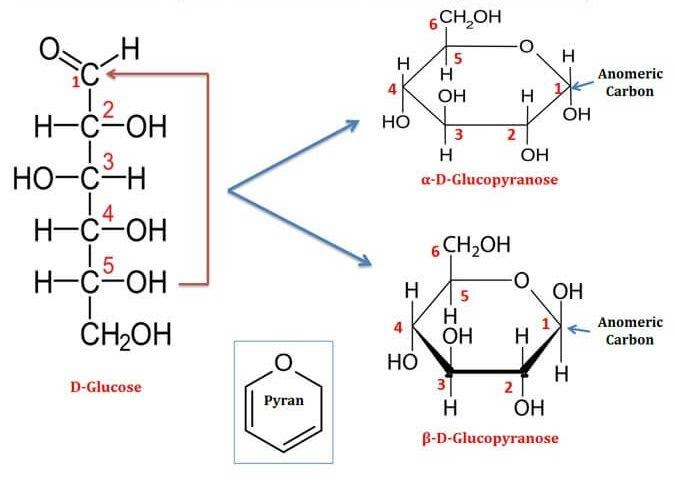
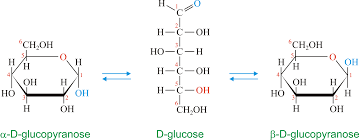
Cyclic Structure Of Glucose
Glucose has 6 carbon atoms and an aldehyde group within it. hence is an aldohexose. Here, the hydroxyl group of the 5th carbon of glucose chain is added to the aldehyde group of the same glucose molecule, resulting in the formation of a cyclic hemiacetal. A pyran ring is created; hence, the structure is a pyranose structure. The atoms present within the ring then arrange themselves in space to minimize the amount of angle strain on each of the covalent bonds. The glucose molecule will attain its most stable configuration when all the carbon atoms of the ring can arrange themselves so that their bond angles are approximately 109.5.
This type of projection is called the Haworth projection of glucose or the Haworth structure of glucose. The single-ring structure of glucose indicates that it is a monosaccharide. Acetals are more prone to basic solutions and nucleophilic attack. Hence, Bending, followed by a rotation of the 4th and 5th carbon bond, of the glucose chain brings the C5-hydroxyl group and the aldehyde groups nearer to each other resulting in the formation of a hemiacetal structure containing a six-membered ring.
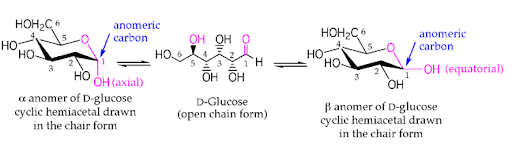
At equilibrium, the β anomer of D-glucose predominates over the alpha anomer, as the hydroxyl group of the anomeric carbon is in the more stable position (equatorial position) of the more stable chair conformation. In alpha-D-glucose, the -OH group on the anomeric carbon is in the axial position of the chair conformation which is the least stable.
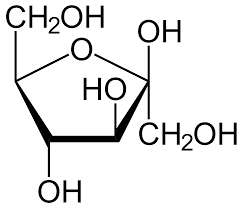
Furanose Structure Of Glucose
When the open-chain form of fructose cyclizes to form a five-membered ring, it is called the furanose structure. When the C-5 hydroxyl group attacks the C-2 ketone of the same molecule, resulting in the formation of an intramolecular hemiketal. Glucose consists of a 6-membered ring, while fructose consists of a 5-membered ring. Hence, the resulting rings are termed furanose (5 members) or pyranose (6 members), respectively, based on their similarity to furan and pyran moieties.
Pyranose Structure Of Glucose
Pyranose is a common term to indicate all the saccharides having a chemical structure that includes a 6-membered ring consisting of five carbon atoms and one oxygen atom within it (pyran moiety should be there). There may be other carbons attached externally to the ring. The 6-membered cyclic structure of glucose is termed a pyranose structure (α or β), in similarity with pyran. Glucose is a six-carbon molecule having an aldehyde functional group which leads to the intramolecular attraction between the carbons and the oxygen atom present in the hydroxyl group of the aldehyde functional group of the same molecule which forces these linear molecules to cyclize into rings. There are a total of 38 conformation structures of this type of pyranose ring. They are 2 chair forms, 6 boat forms, 6 skew boat forms, 12 half-chair forms, and 12 envelopes forms.
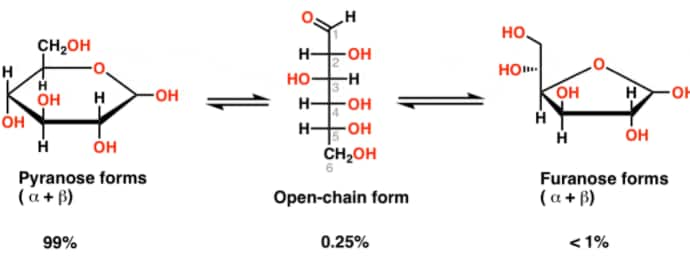
Sugars usually exist in an equilibrium between their cyclic and acyclic form, and this phenomenon is called “ring-chain tautomerism”.
The 6-membered ring is referred to be the “pyranose” and the five-membered ring is “furanose”.
6-membered ring closure generates a stereogenic carbon (chiral carbon,) which is called an anomeric carbon, that leads to the formation of two diastereomers, preferably known as anomers of glucose. They are alpha-D-glucose pyranose; beta-D- glucose pyranose and alpha-D-glucose furanose; Beta-D-glucose furanose
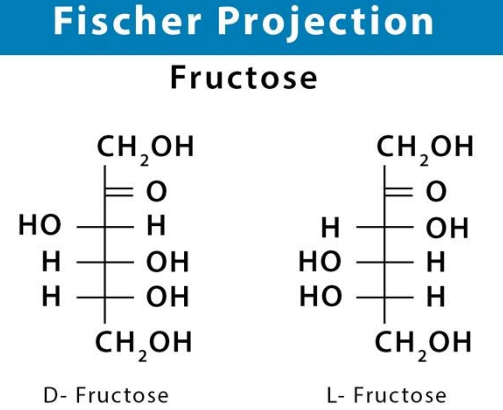
Fischer Projection Of Glucose
Fischer projections depict the structure of sugars in their open-chain form. In this projection technique, the interlinking of the carbon atoms of the sugar molecule is done using solid lines while the interlinking of the C-O and C-H bonds is done horizontally.
Fischer projection Of Fructose
Fisher projections depict the structure of fructose contains a keto group at C-2, and the six carbon atoms are arranged in a vertical line, whereas the hydrogen and -OH groups are arranged horizontally.
Haworth Structure Of Fructose
The Fischer projection of fructose can be converted into the cyclic structure. Like glucose, fructose has a cyclic structure by intramolecular cyclization and results in the formation of alpha and beta anomers.
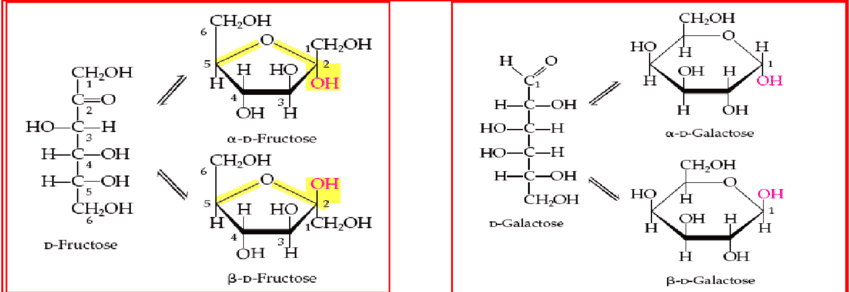
Configuration Of Glucose
In Fischer projection, there are two types that are diastereomers to each other. They are:
In the Haworth projection, there are two types that are enantiomers to each other. They are:
Preparation Of Glucose From Starch
-
Glucose can be obtained as a major product by hydrolysis of starch, at 393 K temperature and 2-3 atm pressure in the presence of dilute sulphuric acid.
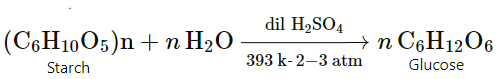
-
Excess sulphuric acid, present in the reaction mixture, is neutralized by adding chalk powder.
-
Activated charcoal is used to remove colored impurities.
-
The resulting solution is then cooled, and crystalline glucose is gradually formed, which is removed by filtration.
Also, students can refer to,
Test of Glucose
A few common tests for the identification of glucose are- Fehling's Test, Benedict's Test, and Barfoed's Test.
-
Fehling's Test: In this test, Fehling's Solution (a deep blue colored solution results when Fehling 1 and Fehling 2 solutions are mixed) is used to identify the presence of reducing sugars and aldehydes.
-
Benedict’s reagent or Benedict’s solution is used to test the presence of reducing sugars. In this test, a glucose solution is heated with Benedict’s solution, and the change in color. It gives positive results for glucose but not for starch.
-
Barford’s test was performed to detect the presence of monosaccharides, hence, sugar. In this test, copper(II) acetate gets reduced to copper(I) oxide (Cu2O) in the presence of aldehyde (aldose sugar), which forms a characteristic brick-red precipitate.
$\mathrm{RCHO}+2 \mathrm{Cu}^{2+}+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{RCOOH}+\mathrm{Cu}_2 \mathrm{O} \downarrow$ (brick red) $+4 \mathrm{H}^{+}$
Also, check
Some Solved Examples
Question 1: Which of the following is $\alpha-\mathrm{D}-\mathrm{Glucose}$ ?
1)
2)
3)
4) None
Solution:
As we have learnt,
Cyclic structure of glucose -
-CHO group is absent, the $-\mathrm{OH}($ at $\mathrm{C}-5)$ group adds to the -CHO group to form cyclic hemiacetal structure.
In the $\alpha$ form of Glucose, the hydroxyl groups at C-1 and C-2 are aligned parallel while in the $\beta$ form, they are anti parallel. It is to be noted that the Carbonyl carbon is converted into the hemiacetal carbon in the cyclic structure of Glucose.
Thus, the structure of $\alpha-\mathrm{D}-\mathrm{Glucose}$ is given as

Hence, the answer is option (3).
Question 2: The following is a structure of?

1) (correct) D-glucose
2) D-Fructose
3) D-Maltose
4) D-Galactose a mixture of (D)-glucose and (L)-glucose
Solution:
As we have learnt,
The given compound represents linear open chain structure of D- Glucose

Hence, the answer is option (1).
Question 3:
The number of chiral carbons in$\beta-D(+)$ -glucose is:
1) (correct) Five
2) Six
3) Three
4) Four
Solution:
Anomers, Epimers, Mutarotation -
Anomers
Anomers are diastereomers that differ in the configuration at the acetal or hemiacetal C atom of sugar in its cyclic form. In other words, anomers are epimers whose conformations differ only about C-1. For example, $\mathrm{a}-\mathrm{D}(+)$ and $\beta-\mathrm{D}(+)$ glucose are anomers.
Epimers
Diastereomers with more than one stereocenter that differ in the configuration about only one stereocenter are called epimers.
- D-glyceraldehyde and L-glyceraldehyde are epimers
- D-Erythrose and L-threose are epimers.
- Epimerization of glucose at C-2 gives mannose.
- Epimerization of glucose at C-3 gives allose.
- Epimerization of glucose at C-4 gives galactose.
Mutarotation
The change in specific rotation of an optically active compound in solution with time, to an equilibrium value, is called mutarotation, or it is the change in the optical rotation occurring by epimerization, i.e., the change in the equilibrium between two epimers, when the corresponding stereocenters interconvert. During mutarotation, the ring opens and then the ring recloses either in the inverted position or in the original position, giving a mixture of α and β forms. All reducing carbohydrates, i.e, monosaccharides and disaccharides, undergo mutarotation in an aqueous solution. Mutarotation proves the existence of anomers and cyclic structures.
There are five chiral carbons in the figure:

Hence, the answer is option (1).
Practice More Questions With the link Given Below
Frequently Asked Questions (FAQs)
Yes, glucose and fructose can be found in the same foods. For example, table sugar (sucrose) is composed of one glucose molecule and one fructose molecule, and many fruits contain both sugars.
Yes, glucose and fructose are structural isomers, meaning they have the same molecular formula (C₆H₁₂O₆) but differ in the arrangement of their atoms.
The main structural difference between glucose and fructose lies in the arrangement of their atoms. Glucose has an aldehyde group (-CHO) and is classified as an aldohexose. It has a six-membered ring (pyranose form) when in solution. In contrast, fructose has a ketone group (C=O) and is classified as a ketohexose. It typically forms a five-membered ring (furanose form) in solution.
The chemical formula for fructose is also C₆H₁₂O₆. Like glucose, fructose is a hexose sugar, but it has a different structure.
The chemical formula for glucose is C₆H₁₂O₆. It is a hexose sugar, meaning it consists of six carbon atoms.
Glucose is made of a six membered cyclic ring made of 5 carbon and one oxygen (pyran ring) and externally added oner carbon containing hydroxyl group.
Yes, glucose and fructose belong to class of sugars. Termed as aldohexose and aldopentose sugar respectively
Glucose is an aldohexose type of sugar
Yes, glucose and fructose have the same chemical formula but they differ geometrically. Hence, they are isomers.
glucose is called dextrose as it is dextrorotatory. It rotates the plane polarized light to the right side.