Glycerol Formula (C3H8O3) - Structure, Applications with FAQs
Introduction
We all know how organic compounds are play an important role in our lives. Organic compounds are chemical combinations of elements; specifically carbon, hydrogen and oxygen. Here, one or more carbon atoms are covalently linked to the atoms of other elements to form a relevant structure. The carbon atoms provide an important structural framework that generates a substantial range of organic compounds.
This Story also Contains
- Glycerol structure:
- Glycerine formula
- Glyceraldehyde formula
- Glyceraldehyde applications
Depending upon the range of combinations formed through various reactions, organic compounds are classified into various categories. They can be alcohols, carboxylic acids, esters, ethers, fats, lipids, etc.
Let us learn about lipids before moving towards glycerol.
Lipids are a class of organic compounds comprising of elements like carbon, hydrogen, oxygen respectively. Lipids are soluble in nonpolar organic solvents but are insoluble in water unlike other organic compounds like alcohols, acids, etc. They are mainly formed of hydrocarbon chains and are usually characterized by their solubility in nonpolar solvents and solubility in water. Lipids are not polymers and hence that lack a monomeric unit but are made up of two molecules:
- Glycerol
- Fatty acids
Glycerol is also referred as glycerine.
Also read -
Glycerol structure:
Glycerol is a colourless, odourless, viscous liquid sweet to taste and non-poisonous. By vicious, it means that the glycerol molecule is liquid having thick and slimy consistency. The glycerol spine is obtained from lipids called as glycerides. Glycerol have antimicrobial and antiviral properties therefore used for burn treatments.
Glycerine formula
The glycerol chemical formula is C3H8O3. The glycerol structure and its appearance are provided below:
Glycerol structural formula and glycerol appearance:-
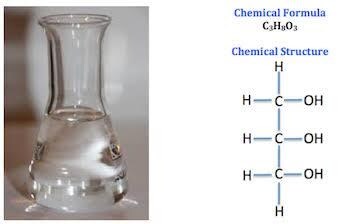
Glycerol is often referred to as glycerin or glycerine where each carbon atom is attached to a hydroxyl group. Glycerol molar mass is 92.09 g/mol. Hence, it is a polyol group as it comprises more than one hydroxyl group. The hydroxyl groups linked to the carbon atoms makes the glycerol molecule water-soluble. These hydroxyl groups are also responsible for their hygroscopic character.
Glycerol, glycerin, trihydroxypropane are the most common terms used to refer the compound. The IUPAC name of glycerol is propane-1,2,3-triol.
This is because of the presence of glycerol (or glycerin) in these products. Glycerol is utilized in skincare and haircare products. Herbal products have herbal extracts as their primary content. While extracting these extracts or fluids from plants and/or herbs the solvent used is glycerol to extract enzymes from the plants or herbs. These extracts are further added to hair care and skin care products.
Glycerol vs Glycerin:
Glycerin chemical formula is the same as glycerol. Glycerol i.e C3H8O3. The main difference between glycerin and glycerol is that glycerol is present in its pure form while glycerin is 95% glycerol. Though the glycerin structural formula and glycerin molecular formula is the same as that of glycerol cannot be preferred interchangeably for their applications in specific areas.
The commercial name for glycerol is glycerin containing 95% of glycerol. Glycerol and glycerin are two different solutions with the same chemical formula. Glycerol is usually used for internal treatments while glycerin is used for external purposes.
The IUPAC name of glycerin is the same as glycerol i.e. propane-1,2,3-triol. The main application of glycerin is in the cosmetic industry. It is widely used as the main ingredient in fragrances, moisturisers and lotions. The main reason to use glycerin in the cosmetic industry is that it provides hydration to the skin, helps in the healing process of minor skin injuries, improves skin mechanical functions, etc.
Glycerin is a humectant form of the moisturizing agent which attracts water from the deeper layers of pores and skin to the uppermost layer of pores and skin which presents hydration to the top layer of pores and skin. Glycerin is secure for intake to sure limits and as it's far used for sweetening, for thickening or as a preservative in several recipes.
Related Topics Link, |
Glycerol emerges as triglycerides and is obtained from plants and animals. Following procedures yield glycerol as a major product:-
- Hydrolysis
- Transesterification
- Saponification
Triglycerides (fats) in the presence of acid and heat or under suitable lipase enzymes are hydrolyzed and yield glycerol and fatty acids.
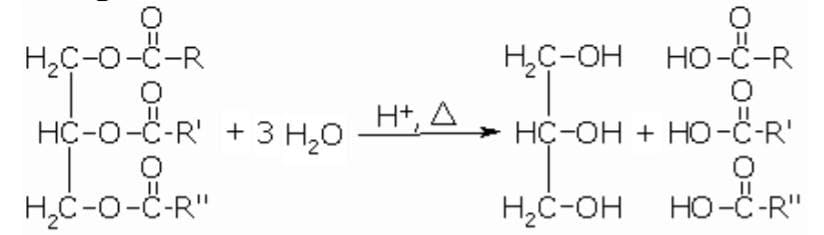
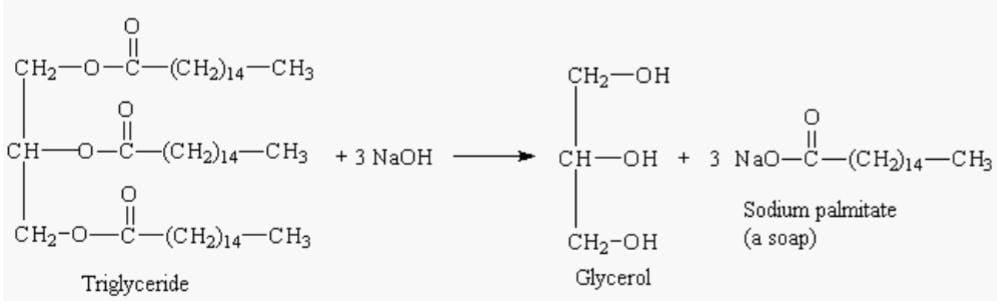
Triglycerides hydrolysis is also carried out in the presence of a base which is usually followed in the industries. Saponification is a process where neutralization and hydrolysis are carried out simultaneously yielding soap.
Also, students can refer,
Glyceraldehyde formula
Glycerol or glyceraldehyde is a triose monosaccharide having formula C3H6O3. Glyceraldehyde is one of the simplest aldoses in its class. It is an intermediate compound in the metabolism of carbohydrates.
Glyceraldehyde is a sweet and colourless solid having a crystalline structure. The IUPAC name of glyceraldehyde is 2,3-dihydroxypropanal. The molecular weight of glyceraldehyde is 90.08 g/mol.
Glyceraldehyde, Glycerol, Glyceric aldehyde are other common terms used to refer the chemical compound.
Glyceraldehyde structure is nothing but glycerol with a hydroxyl group oxidised to aldehyde. Hence the name glyceraldehyde is derived from glycerol and converted to aldehyde through oxidation. The glyceraldehyde structure is given below:
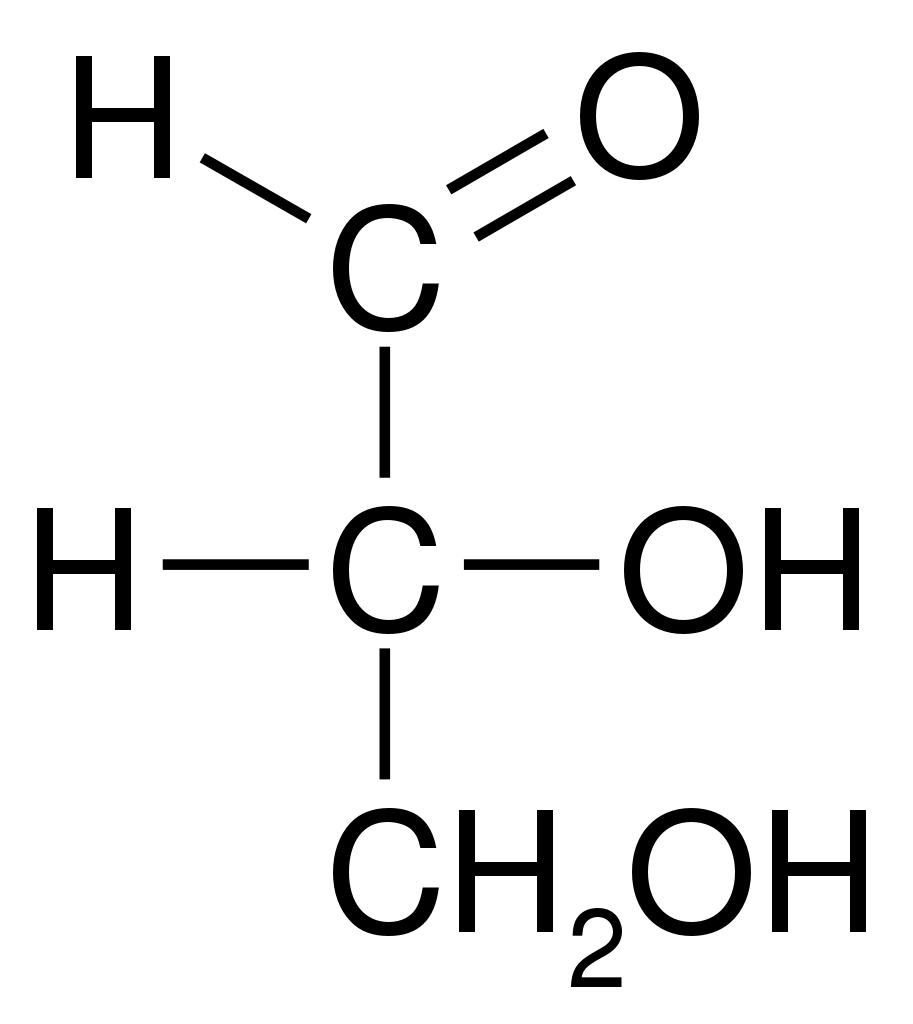
Glyceraldehyde is bonded or linked to four different groups specifying that it has a chiral centre. Therefore it occurs as enantiomers having contrary optical rotation.
The following table represents the enantiomers form of glyceraldehyde:
| D-glyceraldehyde | L-glyceraldehyde | |
| Fisher projection | 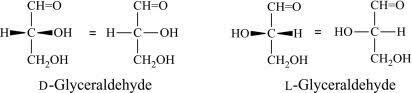 | 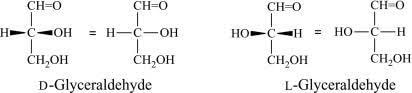 |
The D form and L form of glyceraldehyde are for (R) and (S) enantiomers respectively.
Glyceraldehyde can be prepared by favourable oxidation of glycerol in presence of hydrogen peroxide and the catalyst used is ferrous salt. Dihydroxyacetone is also formed along with glyceraldehyde as they both are isomers of each other.
Glyceraldehyde applications
- Glyceraldehyde is used to prepare polyesters and adhesives.
- Applies as a cellular modifier.
- Used in the tanning of leather.
- D-glyceraldehyde is used as a reference chemical in several biochemical techniques.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: