Lucas Test - Procedure, Observations, Example, Uses, FAQs
Have you ever wondered how chemists differentiate between primary, secondary, and tertiary alcohols in a simple and quick way? The Structure of alcohols significantly influences their chemical behaviour, and how do we identify the structure of alcohol? Well, the answer is the Lucas test, which identifies the structure and reactivity of primary, secondary, and tertiary alcohols.
This Story also Contains
- Lucas Test
- Lucas Reagent
- Procedure Of The Lucas Test
- Observations Of The Lucas Test
- Mechanism Of The Lucas Test
- Uses Of The Lucas Test
- Some Solved Examples

The Lucas test is a qualitative test used to distinguish between alcohols based on their reactivity with the Lucas reagent. It is a mixture of concentrated hydrochloric acid and anhydrous zinc chloride. This test helps to differentiate alcohols by observing how fast the liquid turns cloudy. This happens because alcohols change into insoluble alkyl chlorides on reaction with Lucas reagent. Primary alcohol reacts very slowly or not at all with Lucas' reagent, secondary alcohol shows turbidity after a few minutes, and tertiary alcohols react instantly at room temperature.
Lucas Test
Lucas reagent test involves a reaction in which a substitution reaction takes place. It is based on the rate at which alkyl halides are formed by primary, secondary and tertiary alcohols.
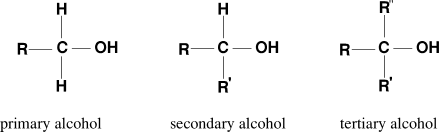
The Lucas test experiment was done in 1930 by Howard Lucas. After that, it is utilized as a standard qualitative analysis in organic chemistry experiments. But due to advancements in the number of spectroscopic and chromatographic analytical methods, this method is not widely used as before and is mainly used for teaching purposes. Lucas test reaction follows SN1 mechanism or unimolecular nucleophilic substitution reaction mechanism where the chloride of the hydrogen chloride, from Lucas reagent, gets replaced by the hydroxyl group of the alcohol.
Carbocation is formed as an intermediate species during a unimolecular nucleophilic substitution reaction or SN1 reaction. Lucas reagent reacts with primary, secondary, and tertiary alcohols to form solutions of different degrees of turbidity. The turbidity of the solution may differ from colorless to turbid. The formation of an alkyl halide (here, chloroalkane) leads to the formation of a turbid solution.
Lucas Reagent
Lucas reagent is a solution of anhydrous zinc chloride (Lewis acid) in concentrated hydrochloric acid. Lucas' reagent is used as a reagent to test alcohols of low molecular weight. Lucas reagent is used to classify alcohols in primary, secondary, and tertiary alcohols according to their reactivity. Lucas reagent is a solution mixture of hydrochloric acid and zinc chloride. To make the Lucas reagent, both of the reacting species, zinc chloride and hydrochloric acid, are taken in equimolar quantities.
The reaction follows a unimolecular nucleophilic substitution reaction or SN1 reaction in which a carbocation intermediate is formed. The stability of this carbocation is the key factor in determining which type of alcohol is used: primary, secondary or tertiary alcohol. Lucas reagent formula: ZnCl2 + HCl; in Lucas reagent, the chloride ion of hydrochloric acid reacts with the alkyl group or substituted alcoholic functional group to form an alkyl halide, while zinc chloride acts as a catalyst.
Procedure Of The Lucas Test
-
The first step is to prepare the Lucas reagent
-
An equimolar amount of zinc chloride and hydrochloric acid is taken to form a solution.
-
Take a small amount of the sample in a test tube that needs to be examined.
-
Add about 2-3 mL Lucas reagent to the unknown sample.
-
Mix the solution well and let it sit.
-
Not the time taken by turbidity to occur.
Lucas' test reaction can be given as:
$ROH \overset{HCl+ZnCl_{2}}{\rightarrow} RCl + H_{2}O$
Observations Of The Lucas Test
The following are observations of the Lucas test if the unknown sample contains:
Primary alcohol: if the unknown sample contains primary alcohol the solution after adding Lucas reagent will not turn turbid at room temperature. However, if the solution is heated for a good 30-45 minutes, turbidity in the solution appears.
Example: C2H5OH + HCl +ZnCl2 room temperature→ NO TURBIDITY
Secondary alcohol: if the unknown sample contains secondary alcohol, the solution after adding Lucas reagent will turn turbid at room temperature after 3-5 minutes.
Example: (CH3)2CHOH HCl+ ZnCl2 (after 3-5 min) → (CH3)2CHCl + H2O + ZnCl2
Turbidity is due to the formation of (CH3)2CHCl.
Tertiary alcohol: if the unknown sample contains secondary alcohol, the solution after adding Lucas reagent will turn turbid at room temperature immediately.
Example: (CH3)3COH HCl+ ZnCl2→ (CH3)3CCl + H2O + ZnCl2
|
Related Topics, |
Turbidity is due to the formation of (CH3)3CCl.Lucas reagent reacts with primary, secondary, and tertiary alcohols through a unimolecular nucleophilic substitution reaction mechanism and forms a carbocation as an intermediate with all three alcohols. As we know, the stability of the carbocation intermediate is 3°>2°>1°. Lucas reagent gives instant turbidity with tertiary alcohol due to the formation of a highly stable 3° cation.
While secondary alcohol forms a moderately stable 2° carbocation intermediate and gives a result with Lucas reagent after 3- 5 minutes. Primary alcohol, on the other hand, shows no signs of turbidity at room temperature when the Lucas reagent is added to the test tube. This is because primary alcohol reacts with Lucas reagent to form a 1° carbocation intermediate, which is highly unstable.
Mechanism Of The Lucas Test
Lucas reagent reacts with alcohol through a unimolecular nucleophilic substitution reaction mechanism or SN1 reaction mechanism. The overall mechanism takes place in two steps:
1. Formation Of Carbocation Intermediate
The OH group belonging to the alcohol is protonated by hydrochloric acid. Since chlorine is a stronger nucleophile than water, it replaces the resulting water molecule attached to the carbon. This leads to the formation of a carbocation.
2. Nucleophilic Attack
This step involves an attack of a chloride ion, which is a nucleophile, on the carbocation intermediate to form alkyl chloride. The hydrogen ion form due to the ionization of hydrogen chloride, reacts with the leaving hydroxyl groups to form water. Zinc chloride, being a catalyst, gets removed unaffected.

Uses Of The Lucas Test
Most important application of the Lucas test is to distinguish between primary, secondary and tertiary alcohols using Lucas reagent.
|
Type of alcohol present in the sample |
Reaction with Lucas reagent |
Observation after adding Lucas reagent |
Carbocation formed |
|
Primary alcohol |
C2H5OH + HCl +ZnCl2 (Lucas reagent) room temperature→ No change |
No turbidity in the solution or colorless solution, as no reaction takes place at room temperature. |
Primary carbocation (highly unstable) |
|
Secondary alcohol |
: (CH3)2CHOH HCl+ ZnCl2 lucas reagent (after 3-5 min) → (CH3)2CHCl + H2O + ZnCl2 |
Turbid solution after 3- 5 min. A white, cloudy solution formed at room temperature. |
Secondary carbocation (moderately stable) |
|
Tertiary alcohol |
(CH3)3COH HCl+ ZnCl2 lucas reagent → (CH3)3CCl + H2O + ZnCl2 |
Turbid solution instantly at room temperature. A white, cloudy solution formed immediately. |
Tertiary carbocation (highly stable) |
Also, students can refer,
Some Solved Examples
Question 1. Lucas reagent is a mixture of:
a) Conc. $\mathrm{HCl}+\mathrm{CuCl}_2$
b) Conc. $\mathrm{HCl}+\mathrm{ZnCl}_2$
c) Dil. $\mathrm{HCl}+\mathrm{ZnCl}_2$
d) $\mathrm{HCl}+\mathrm{H}_2 \mathrm{SO}_4$
Solution:
Lucas reagent consists of concentrated HCl and anhydrous ZnCl₂, used to differentiate alcohols based on their reactivity.
Hence, the correct answer is option (b)
Question 2: The Lucas test is primarily used to distinguish between
a) Alkanes and alkenes
b) Primary, secondary, and tertiary alcohols
c) Aldehydes and ketones
d) Acids and esters
Solution:
Lucas test identifies the class of alcohol based on the speed of alkyl chloride formation.
Hence, the correct answer is option (b) Primary, secondary, and tertiary alcohols
Question 3: Which alcohol gives an immediate cloudy appearance in the Lucas test?
a) Methanol
b) Ethanol
c) 2-Propanol
d) 2-Methyl-2-propanol (tert-butanol)
Solution:
Tertiary alcohols react fastest with Lucas reagent, forming insoluble alkyl chlorides immediately. And 2-Methyl-2-propanol is a tertiary alcohol. So it gives a cloudy appearance immediately
Hence, the correct answer is option (d)
Practice More Questions With The Link Given Below
Also read -
Frequently Asked Questions (FAQs)
The test works by reacting an alcohol with Lucas reagent. Tertiary alcohols react almost immediately to form a cloudy solution because they quickly convert to tertiary alkyl halides. Secondary alcohols take a few minutes to react, while primary alcohols typically do not react at room temperature, demonstrating a clear differentiation among the alcohol types.
The main components of Lucas' reagent are zinc chloride and hydrochloric acid.
Carbocation formation is crucial because the stability of the carbocation determines the reaction rate. Tertiary alcohols form the most stable carbocations, leading to a rapid reaction, while primary alcohols form less stable carbocations, resulting in slower or negligible reactions.
Lucas Test limitation
- Its inability to differentiate between secondary and tertiary alcohols, which may behave similarly under certain conditions.
- The test does not work well for some sterically hindered primary alcohols or for compounds that do not produce stable carbocations.
