Methods of Separation - Definition, Mixtures, FAQs
What is Separation?
A separation is a process where the compounds of interest are removed from the other compound in a sample that may or may not react similarly and interfere with a quantitative determination. The process of separation is done to remove unrequired component(s) or obtain desired component(s) from a mixture. In other words, done for separating components of a mixture. Separation techniques are used in the preparation of pure compounds and almost every chemical substance we see around us. A common example is separation of sand and water. A mixture of sand and water can be separated by filtration method.
Some methods of separation of substances are discussed below:
Winnowing
This is a separation process in which the mixture of substances is blown in air so that the lighter substances get dispersed by wind and the heavy substances can be separated. Example- separation of grain from husk is done by farmers using winnowing shovels. Winnowing drawing is given below:
Threshing
Threshing is one of the methods of separation involving beating the stalk of grains against an object to separate grains from stalks. This process is typically used in farming.
Sieving
Sieving is a separation process in which a semi porous barrier (or sieve) is used to separate the mixture to obtain the required portion of mixture. Example- Sieves are used to remove impurities from flour.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Handpicking
Handpicking is a separation process involving selective picking of unwanted particles by hand. Example- picking marbles from a bucket of water.
Magnetic Separation Method
This method is used in separating components of a mixture of magnetic substances or mixtures containing magnetic components. In this method, the substance which needs to be separated is passed through a conveyor belt attached to magnetic rollers. The magnetic rollers attract the magnetic portion of the substance which is collected in separator drums. In this way, the magnetic component(s) is separated from the non-magnetic component(s). This is often used in separation of solid solid mixture. Magnetic Separation images are given below:
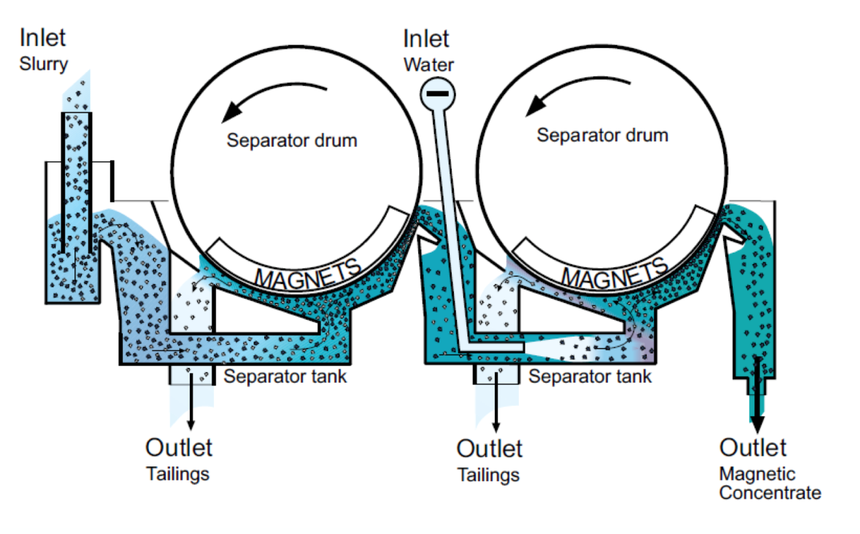
Other common methods of separation are sedimentation, evaporation and filtration.
Separation Techniques for Mixtures
Separations are complex and may require several different methods and much time before the final determinative step. The separation process used depends on the component of the mixture and their characteristic properties.
There are various methods of separating mixtures, they can be categorized as:
Separations involving phase changes
Separations involving extraction
Separations involving chromatography
Separations involving ion exchange resins
Separations involving floatation
Separation Involving Phase Change
There are 3 phases of matter that we see in everyday life namely solid, liquid and gas. When a liquid changes into a gas, this gas is called a vapor. When a component of a mixture can undergo phase change, separating components of a mixture becomes easier. The simplest separation process example is the removal of water from any solid substance. When the substance is heated the water (liquid) changes to vapor and separates from the solid compound. This separation process is called volatilization. Another important method for separating the components of a mixture is distillation. Distillation is a separation process in which vapor is produced by heating a liquid in a vessel. The vapors are then condensed followed by collection in another vessel.
Related Topics, |
Separations Involving Extraction
An extraction is a separation process involving the selective transfer of a compound(s) from one liquid to another immiscible liquid (liquid-liquid extractions) or from a solid to a liquid (solid-liquid extraction).
Separating Funnel Method
Separating funnel method is a common method used for separating the components of a mixture. A separatory funnel is the simplest type of extraction vessel. This method of separation is utilized to separate two immiscible liquids. Example - Oil and water mixture. Separating funnel diagram is given below:
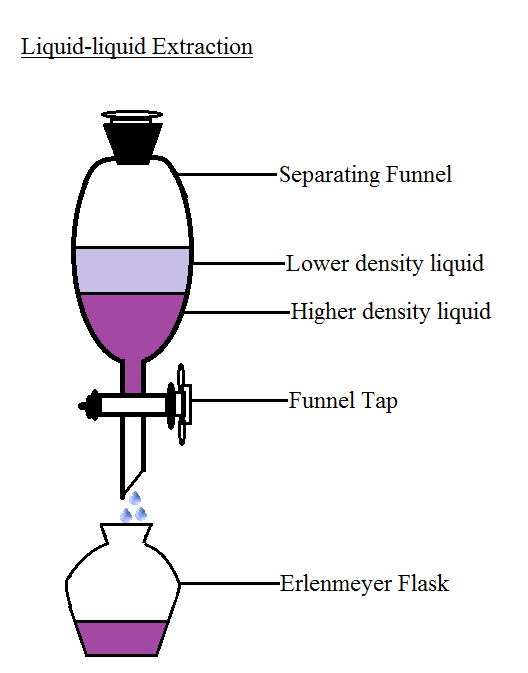
Image of separating funnel
Separations Involving Chromatography
Chromatography is one of the multistage separation techniques based on the principle of different rates between compounds in adsorbing on a surface or dissolving in a thin film of liquid. The two major chromatographic separation techniques at work used for separating the components of mixture are displacement and partition chromatography. Other common chromatographies are paper, thin layer, high performance, gas chromatography and gel permeation.
Also, students can refer,
- NCERT Solutions for Class 12 Chemistry Chapter 6 General Principles and Processes of isolation of
elements - NCERT Exemplar Class 12 Chemistry solutions Chapter 6 General Principles and Processes of isolation of
elements - NCERT notes Class 12 Chemistry Chapter 6 General Principles and Processes of isolation of elements
Separation Involving Ion Exchange Resins
Ion exchange is a separation process used for separating mixtures in which one type of ion in a compound is exchanged for a different type i.e., cation for cation and anion for anion. For example, calcium, iron and magnesium ions can be removed from water by using sodium ions.
Separation Involving Flotation
Flotation is a separation process performed by using a gas to cause selected solid particles to rise to the surface from a mixture of particles suspended in a liquid. Flotation without involving a gas can be done by using a liquid that will selectively coat the solid particles.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: