Ionic bonds form when atoms (typically metals and nonmetals) exchange electrons to create oppositely charged ions that attract each other.
Covalent bonds involve the sharing of electron pairs between atoms, enabling them to complete their valence shells.
Coordinate (dative) covalent bonds occur when both electrons in a shared pair originate from the same atom, such as in metal-ligand complexes.
Metallic bonds arise in metal solids, where valence electrons delocalize among positive ions, providing conductivity and malleability .
Hydrogen bonds are strong dipole-based attractions between H bound to electronegative atoms (N, O, F) and lone-pair-bearing atoms.
Vander- Waals forces (including dipodipole and London dispersion interactions) are weaker attractions between molecules.
Multiple bonds (double and triple bonds) consist of sigma (σ) and pi (π) overlaps and strengthen bonding by increasing bond order.
Chemical Bonding and Molecular Structure - Notes, Topics, Books, FAQs
Chemical bonding explains how atoms connect to form stable molecules by lowering their overall energy. It examines why certain atoms combine and arrange themselves into specific shapes. Key theories include VSEPR, which predicts molecular geometry based on electron pair repulsion; Valence Bond Theory, which describes bond formation through overlapping atomic orbitals and hybridization; and Molecular Orbital Theory, which constructs molecule-wide orbitals to account for bond order, stability, and properties like magnetism. Bonding reflects nature’s drive to reach the most stable electronic configuration.
This Story also Contains
- Chemical Bonding And Molecular Structure Important Topics
- Overview of Chemical Bonding
- How to Prepare for Chemical Bonding?
- Chemical Bonding and Molecular Structure in Different Exams:
- Study Links for Chemical Bonding and Molecular Structure
- Subject Wise Resources of NCERT
- Previous Year Questions of Chemical Bonding And Molecular Structure
- Prescribed Books
In our body itself, all the macromolecules like DNA, RNA, Proteins, etc are held together with the help of this chemical bonding. All the structures are held together with the help of this chemical bond, either the bond is stronger or weaker. Based on the strength of the bonds, the stability of the structure is determined. The melting and boiling point, etc are the important properties that are determined by the strength of the chemical bond. This chapter of Chemistry is usually liked by every student as it is very easy and it holds enough distribution of marks in Board exams and other competitive exams like JEE and NEET.
Chemical Bonding And Molecular Structure Important Topics
In this section, you will study the important topics of the chapter, overview, formulae, and some important tips and guidelines for the preparation of the chapter at the best.
Ionic bonding
Ionic bonds or Electrovalent bonds are formed from the electrostatic attraction between oppositely charged ions in a chemical compound.
Fazan’s rule:
Fajan’s Rule helps to predict whether a chemical bond is ionic or covalent. It states that the size and charge of cations and anions determine the type of bond forms.
Lewis Electron Dot Structures:
Lewis Electron Dot Structures is an important topic in this chapter, where we study how electrons are arranged in elements to form bonds with other elements.
Rules for Drawing Lewis Structures
-
Write the chemical symbol of the element.
-
Determine the number of valence electrons:
-
For main group elements, valence electrons = group number.
Example: Oxygen (Group 16) → 6 valence electrons.
-
-
Place dots around the symbol to represent valence electrons:
-
One dot per electron initially, then pair them to complete octets if needed.
-
Maximum of 2 electrons per side (top, bottom, left, right) around the symbol.
-
-
Form bonds (if showing molecules):
-
A single bond represents a shared pair of electrons between two atoms.
-
Double or triple bonds are used to satisfy the octet rule for elements like $\mathrm{O}_2, \mathrm{~N}_2, \mathrm{CO}_2$.
-
Limitations Of The Octet Rule:
Limitations Of The Octet Rule is also an important topic. The octet rule has various limitations it does not apply to all states, it does not count the number of electrons, and it does not apply to elements with fewer than eight electrons.
Formal charge and Lewis dot structures:
A hypothetical charge assumed to be an atom in a molecule is called a formal charge, assuming that electrons are shared equally between atoms. It is very helpful in predicting a molecule's reactivity and structure.
Bond Parameters - Bond Order, Angle, Length, And Energy:
Bond Parameters - Bond Order, Angle, Length, And Energy is the most important topic for knowing the stability of the bond or the compounds form after bonding.
Valence Shell Electron Pair Repulsion (VSEPR) theory:
Vsepr Theory is based on the repulsion between pairs of valence electrons. The electrons repel each other in order to minimize the repulsion.
Valence Bond Theory (VBT):
The overlap between two incompletely filled atomic orbitals leads to the formation of a bond between two atoms is called Valence Bond Theory.
Hybridization and its types:
The process of combining two atomic orbitals to create a new type of hybridized orbitals is called Hybridisation is also an important topic.
Molecular Orbital Theory (MOT):
Molecular Orbital Theory explains how molecules form and behave by describing the distribution of electrons in a molecule. It has a different principle which tells molecular orbitals are equal to the atomic orbitals.
Also read,
- Formation of Ionic Compounds
- Lattice Energy
- Drago's Rule
- Pi Bonds
- Molecular Geometry
- Dipole Moment
- Van Der Waals Forces
- Hydrogen Bonding
Overview of Chemical Bonding
All atoms in this universe seek to achieve their stable electronic configuration in the “Noble gas” configuration. Atoms except Hydrogen achieve this stable noble gas configuration by following the octet rule in the following ways:
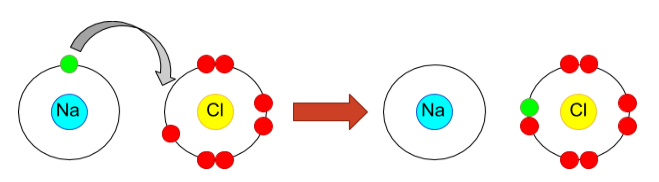
(i) Ionic bond: In this type of bond, metals and non-metals take part in the bond formation by the complete exchange of electrons as depicted in the picture below:
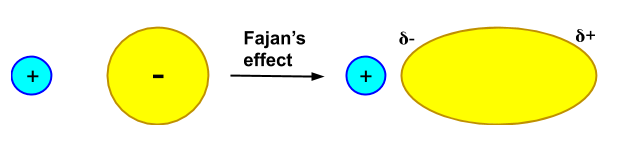
(ii) Fazan's Rule: This rule states that in every ionic bond, there is always some percentage of covalent character. This covalent character depends upon the size of the anion and cation, the charge of the anion and cation. As the charge on the cation is larger and its size is smaller its polarising power increases and thus the covalent character increases. Similarly, for an anion, if its charge is higher and its size is also larger, then its polarisability increases and thus the covalent character increases.
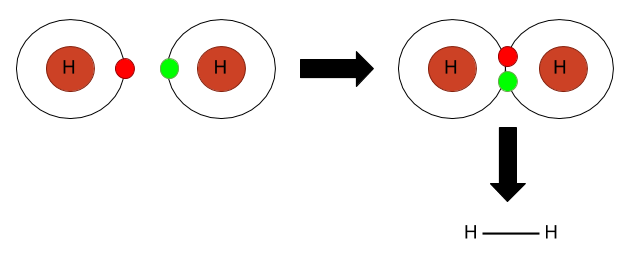
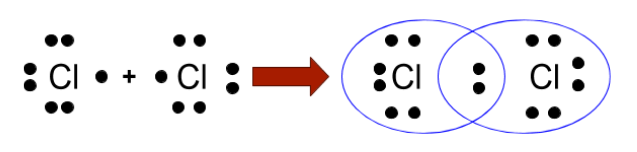
(iii) Covalent bond: This type of bond formation takes place by the sharing of electrons between the non-metals. The picture given below shows its representation.
In this chapter, you will learn about the Lewis dot structures. Lewis dot structure is the simple representation of the molecules formed by the covalent bonds. However, in this concept, the geometry of the molecules needs to be determined, which is one of the major drawbacks of this concept. So to overcome these drawbacks, several advanced theories have been given by scientists, and they are VSEPR, VBT, and MOT.
(iv) Kossel Lewis Approach: This is the simplest model for explaining the structures of the chemical bond. This approach considers the central atom as a sphere and electrons as point charges around it. Now, these point charges electrons are shared between atoms or completely exchanged between atoms and form the covalent and ionic bond respectively. After the formation of these bonds, all the atoms engaged in bonding achieve the stable noble gas configuration.
(v) VSEPR Theory:
-
In VSEPR theory, it was said that the shapes of the molecules are determined by the combination of a lone pair of electrons and bond pair electrons.
-
These electrons repel each other. In order to minimize the repulsion, these electrons arrange themselves at the maximum distance and hence form the geometry.
-
Some common geometries of the molecules are tetrahedral, square planar, octahedral, etc.
(vi) Valence Bond Theory:
-
The bond formation takes place only by the valence orbitals containing unpaired electrons and spinning in opposite directions.
-
Bond formation takes place by partial overlapping of orbitals. So, according to this theory, various kinds of overlapping occurs during bond formation like s-s overlapping, s-p overlapping, p-p overlapping, and pi bond (lateral overlapping of p orbital).
(vii) Hybridization:
- This concept says that during the time of bond formation, the first intermixing of orbitals of comparable energies occurs to produce the new orbitals, also known as hybrid orbitals, and then bond formation occurs. The hybridization concept eliminates all the drawbacks that were not solved by the earlier theories.
- The number of hybrid orbitals produced is equal to the number of atomic orbitals.
- The hybrid orbitals are identical in shape, size, energy, etc.
- The hybrid orbitals always form a sigma bond.
For example, $\mathrm{BeF}_2$. This bond is not explained by VSEPR and VBT, thus hybridization explains its bond formation and structure. This is explained below:
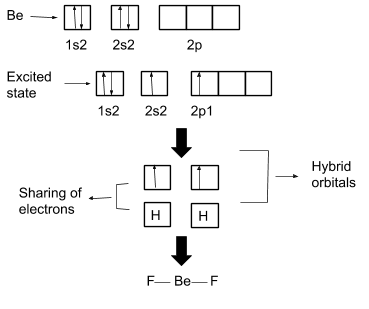
Finally, at the end of this chapter, you will learn about a new theory i.e. “Molecular Orbital Theory”. This theory is applicable to only diatomic molecules like $\mathrm{O}_2, \mathrm{~N}_2, \mathrm{~F}_2$,etc. This theory explains several things like electron distribution in bonding and antibonding molecular orbitals, bond order, and paramagnetic or diamagnetic nature of molecules.
How to Prepare for Chemical Bonding?
Though classified under physical chemistry, this chapter is entirely conceptual no formulas or number-crunching needed. A solid grasp of topics like atomic structure and elemental periodicity is essential before tackling it these foundations inform your understanding of bonding. After mastering the theory, reinforce your learning by attempting a mock test. It’s especially helpful to meticulously review your Class 11 NCERT textbook, as it offers the clarity and depth necessary for strong conceptual retention.
- Students must understand the basic concepts first like octet rule, Lewis symbols, and ionic bond formation.
- Learn how lattice energy and Born Haber cycle explains stability of ions.
- Then understand the covalent bonding, theories, Lewis structures, resonance, and formal charge.
- Questions related to Valence Bond Theory and Hybridisation are often asked in exams to understand these concepts better refer to class 11 chemistry chapter 4 chemical bonding and molecular structure notes.
- Also read molecular shape and geometry in detail for predicting molecular geometry.
- Learn about molecular orbital theory, bond order, stability, magnetism of molecules, polarity and intermolecular forces
- At last students can solve previous year questions from this chapter.
Chemical Bonding and Molecular Structure in Different Exams:
This chapter is widely asked in board and competitive examinations with focus on bond formation, molecular geometry, hybridisation, and theoretical models explaining chemical bonding.
| Exam Name | Focus Area | Common Topics Asked | Preperation Tips |
| CBSE Board | Conceptual understanding | VSEPR theory, hybridisation | Practise diagrams and examples |
| JEE Main | Concept application | Bond parameters, hybridisation | Solve MCQs regularly |
| JEE Advanced | Analytical understanding | MOT, shape prediction | Focus on theory and reasoning |
| NEET | NCERT-based theory | Bond types, shapes | Revise NCERT thoroughly |
| State Board Exams | Theory-oriented | Definitions, bond types | Learn key terms and diagrams |
| Chemistry Olympiads | Advanced application | MOT, polarity, intermolecular forces | Practise high-level problems |
Study Links for Chemical Bonding and Molecular Structure
This section provides useful study resources and reference links to help you understand the fundamentals and advanced concepts of chemical bonding and molecular structure effectively.
Subject Wise Resources of NCERT
This section provides organised study materials and useful links for each subject to support effective learning and exam preparation.
Previous Year Questions of Chemical Bonding And Molecular Structure
This section includes important questions frequently asked in previous examinations, covering key concepts of chemical bonding and molecular structure. Practicing these questions helps in understanding exam patterns and strengthening conceptual clarity.
Question 1: Given below are two statements:
Statement (I) : For ClF3, all three possible structures may be drawn as follows.
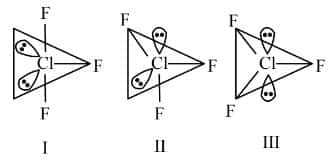
Statement (II) : Structure III is most stable, as the orbitals having the lone pairs are axial, where the bond pair repulsion is minimum.
In the light of the above statements, choose the most appropriate answer from the options given below:
1. Statement I is incorrect but statement II is correct.
2. Statement I is correct but statement II is incorrect.
3. Both Statement I and statement II are correct.
4. Both Statement I and statement II are incorrect.
Answer:
Statement 1 is correct.
Statement 2 is incorrect since in sp3d hybridization, a lone pair cannot occupy an axial position due to lone pair-bond pair repulsion. The lone pairs will be at equatorial position for maximum stability.
Hence, the correct answer is option (2).
Question 2: Which of the following statements are not correct?
1) NaCl being an ionic compound is a good conductor of electricity in the solid state.
2) In canonical structures there is a difference in the arrangement of atoms.
3) Hybrid orbitals form stronger bonds than pure orbitals.
4) VSEPR Theory can explain the square planar geometry of $X e F_4$
Answer:
NaCl, being an ionic compound is a good conductor of electricity in solid state and (2) In canonical structures, there is a difference in the arrangement of atoms
Explanation: In (i) ions are not free in solid state but are strongly bonded through electrostatic forces, and in (ii) the arrangement of atoms is not different in the cannonical form (resonance form). The delocalization of electrons takes place entire the molecule but the position of atoms remains the same.
Hence, the answer is option (1).
Question 3: Which of the following statements are correct about $\mathrm{CO}_3{ }^{2-}$?
(i) The hybridization of central atom is sp3.
(ii) Its resonance structure has one C–O single bond and two C=O double bonds.
(iii) The average formal charge on each oxygen atom is 0.67 units.
(iv) All C–O bond lengths are equal.
1) (i) and (iii)
2) (ii) and (iv)
3) (iii) and (iv)
4) None of above
Answer:
The answer is the option (iii) The average formal charge on each oxygen atom is 0.67 units and (iv) All C-O bond lengths are equal.
Explanation: In resonating structures bonds are not fixed, and as a result, all bond lengths are equal.
Hence, the answer is the option (3).
Practice more questions from the link given below:
Prescribed Books
For chemical bonding and molecular structure, first, you need to finish the theory thoroughly from the NCERT book and then solve the examples and questions given in the book. Apart from this, if you want to prepare for the advanced level of competitive exams like JEE and NEET, you must read the book - O.P Tandon or P. Bahadur. Meanwhile, in the preparation, you must continuously take the mock tests for internal assessment.
Frequently Asked Questions (FAQs)
Ionic bonds occur when electrons are transferred from one atom to another, typically between metals and nonmetals, resulting in the formation of charged ions. Covalent bonds involve the sharing of electron pairs between atoms, usually between nonmetals, leading to the formation of molecules.
Molecular geometry, which describes the three-dimensional arrangement of atoms in a molecule, influences properties such as polarity, boiling and melting points, and reactivity. For example, molecules with a symmetrical shape are often non-polar, while those with asymmetrical shapes may be polar.
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the geometry of molecules based on the idea that electron pairs around a central atom repel each other and will arrange themselves to minimize this repulsion. The arrangement of these electron pairs determines the shape of the molecule.
Electronegativity is a measure of an atom's ability to attract and hold onto electrons. In a chemical bond, the difference in electronegativity between two atoms can determine whether the bond is ionic, polar covalent, or non-polar covalent. Greater differences typically lead to ionic bonds, while smaller differences lead to covalent bonds.
Resonance refers to the concept that some molecules can be represented by two or more valid Lewis structures, known as resonance structures, which differ only in the placement of electrons. The actual structure is a hybrid of these resonance forms, contributing to the stability and properties of the molecule.
