Sulfur - Overview, Properties, Applications, Uses, FAQs
What is Sulphur (s)
S (symbol)
Atomic number of sulphur or sulphur atomic number: 16 Atomic mass: 32.065 u
[Ne] 3s23p4 is the electron configuration
Electrons per shell is 2,8,6
2.58 electronegativity
115.2 °C is the melting point
Sulphur meaning
Sulfur element, also written sulphur, is a non-metallic chemical element that belongs to the oxygen group of the periodic table (Group 16)
It is a very reactive element. Pure sulphur is a brittle, tasteless, odourless, pale yellow solid that is water insoluble and a poor conductor of electricity.It forms sulphides with all metals except gold and platinum; it also forms compounds with a number of non-metallic elements. Each year, millions of tonnes of sulphur are manufactured, largely for the production of sulfuric acid, a widely used industrial chemical.
Sulfur is the 10th most plentiful element in the universe, accounting for one atom in every 20,000–30,000.Although sulphur is categorised as a small ingredient of Earth's crust, its proportion is estimated to be between 0.03 and 0.06 percent, it occurs in the uncombines condition as well as in combination with other elements in widely distributed rocks and minerals. It has been suggested that deeper layers of Earth contain a substantially bigger proportion of sulphur based on the discovery that certain meteorites contain roughly 12% sulphur. In the form of sulphate, seawater contains around 0.09 percent sulphur.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Powder, brimstone, or various spellings
Because sulphur is one of the few elements that exists in its purest form, mankind has long known about and used ‘pure' elemental sulphur. Sulfur was used in traditional Chinese medicine about 2,600 years ago, according to records. By the 7th century AD, they had figured out how to make black powder by combining sulphur, charcoal, and potassium nitrate (gunpowder). Sulfur was also employed in ancient Indian, Greek, and Egyptian societies for healing, fumigation, and fabric bleaching.
Sulfur is also mentioned in religious texts dating back 2,600 years. Sulfur is referred to as 'brimstone' in English Bible translations to describe damage (as volcanic activity is capable of doing) and the foul odour associated with sulphur compounds.
Let's fast forward 2,300 years to the year 1777. Antoine Lavoisier discovered that he couldn't break sulphur down into simpler chemicals after many experimentations, thus he labelled it an element.
occurrence and distribution in nature
Many major metal ores are sulphur compounds, such as sulphides and sulphates. Galena (lead sulphide, PbS), blende (zinc sulphide, ZnS), pyrite (iron disulphide, FeS2), chalcopyrite (copper iron sulphide, CuFeS2), gypsum (calcium sulphate dihydrate, CaSO4.2H2O), and barite (calcium sulphate dihydrate, CaSO4.2H2O (barium sulfate, BaSO4). Although a procedure devised in the 18th century for manufacturing sulfuric acid used sulphur dioxide generated by burning pyrite, sulphide ores are prized primarily for their metal content. Sulfur compounds can be found in coal, petroleum, and natural gas.
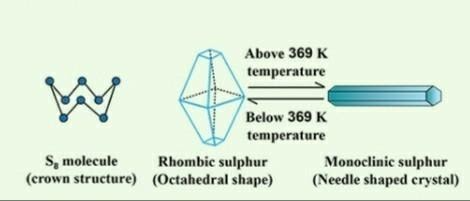
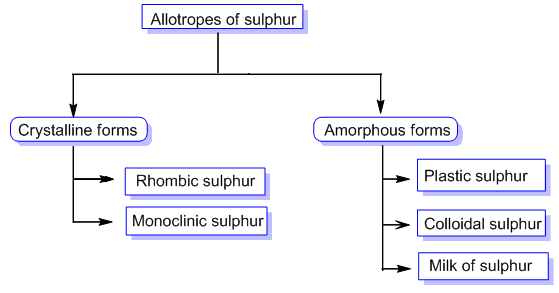
Sulphur Allotropy
Sulphur and Its Allotropes: An Overview
Sulphur and its allotropic forms are discussed in this topic. Sulphur is found in group 16 of the periodic table and makes approximately 0.17 percent of the earth's crust. It's a non-metal created as a by-product of natural gas extraction.
The Sulphur Allotropes
Sulphur exists in several allotropes, but we'll concentrate on the two most common: yellow rhombic sulphur (beta-sulphur) and monoclinic sulphur (beta-sulphur). The most intriguing attribute is their thermal stability; the sulphur allotropes are interconvertible, that is rhombic sulphur becomes monoclinic sulphur when warmed over 369 K.Let's take a closer look at these two allotropes.
Sulphur with a rhombic shape is known as rhombic sulphur (beta-sulphur).
Rhombic sulphur is crystalline and has an octahedral structure. We get rhombic sulphur when we heat a roll sulphur solution in CS2. It has a melting point of 385.8K and a specific gravity of 2.06. Rhombic sulphur is not water soluble, although it is soluble in benzene, ether, alcohol, and other solvents.
Monoclinic sulphur (β -sulphur) is a type of sulphur that is monoclinic in nature.
Monoclinic sulphur is produced by melting rhombic sulphur in a dish and then cooling it. In this approach, we make two holes in the crust and drain the remaining liquid. When the crust is removed, we receive colourless needle-shaped crystals of β-sulphur.
Sulphur’s applications
Because sulphur is so widely employed in industrial processes, it is frequently considered as a trustworthy predictor of industrial activity and the state of the economy. Approximately six-sevenths of all sulphur generated is processed into sulfuric acid, which is used mostly in the fertiliser industry (phosphates and ammonium sulfate). Pigments, soaps, fibres, petroleum, metal sheets, explosives, and storage batteries are only a few examples of notable applications. Sulphur that has not been converted to sulfuric acid can be found in a variety of items such as paper, pesticides, fungicides, dyestuffs, and a variety of other chemicals.
Properties of sulphur
Sulfur can be found in a variety of polyatomic compounds with various chemical formulae. Octasulfur, S8, is the most well-known allotrope, which is a soft, yellow solid with a mild odour. At high temperatures, it experiences phase shifts from octasulfur to beta-polymorph to sulfur due to alterations in intermolecular interactions.
Depolymerization occurs at temperatures over the boiling point of octasulfur. Above 200°C, molten sulphur turns a dark red colour. The density of different allotropes varies by around 2 g/cm3. Electrically, stable allotropes are excellent insulators.
Sulphur emits a blue flame and produces sulphur dioxide, which has a suffocating odour. It's water insoluble, but it's soluble in carbon disulfide. S+4,S6+, and S2+ are more common. Only powerful oxidants like fluorine, oxygen, and chlorine have higher ionisation states.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 7 The P-block elements
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 7 The P-block elements
- NCERT notes Class 12 Chemistry Chapter 7 The P-block elements
Sulfur Chemical Properties
Sulfur emits a blue flame, which is accompanied by the creation of sulphur dioxide, which has a unique suffocating odour. Sulfur dissolves in carbon disulfide and, to a limited extent, apolar organic solvents such as benzene and toluene, but not in water.
The first and second ionisation energies of sulphur are 999.6 and 2252 kJ/mol, respectively. Despite these numbers, S2+ is rather uncommon, with S+4 and S6+ being more common. 4556 and 8495.8 kJ/mol are the fourth and sixth ionisation energies, respectively. Electron transport across orbitals causes the magnitude of the figures; these states are only stable with strong oxidants like fluorine and oxygen.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:

