Potential Energy Of A Dipole In An Electric Field
The potential energy of a dipole in an electric field is a concept that bridges fundamental physics with real-life applications. Imagine holding a bar magnet near a fridge; the magnet aligns itself due to the fridge’s magnetic field, minimizing its potential energy. Similarly, a dipole—consisting of two equal and opposite charges separated by a distance—experiences forces in an electric field, leading to a change in its orientation and potential energy. This concept is crucial in understanding molecular behaviour, designing electronic devices, and even in medical imaging techniques like MRI, where dipoles in atoms interact with external fields. In this article, we will understand the Potential energy of a dipole in an electric field. it helps explain how systems tend to move towards states of lower energy, a principle observed in many natural and engineered processes.
This Story also Contains
- Potential Energy of a Dipole in an Electric Field
- Equilibrium of Dipole
- Solved Examples Based on Potential Energy of a Dipole In an Electric Field
- Summary
Potential Energy of a Dipole in an Electric Field
The potential energy of a dipole in an electric field is a fundamental concept in electromagnetism that has wide-ranging implications in both natural phenomena and technological applications. A dipole, which consists of two opposite charges separated by a distance, interacts with an external electric field in a way that causes it to align with the field. This alignment results in a change in the dipole's potential energy, depending on the angle between the dipole moment and the electric field.
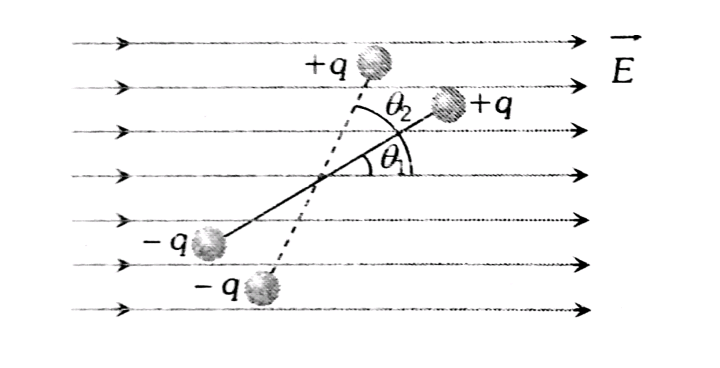
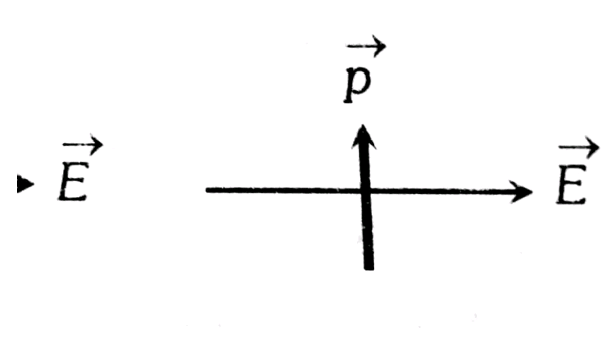
When a dipole is kept in a uniform electric field. The net force experienced by the dipole is zero as shown in the below figure.
I.e

But it will experience torque. The net torque about the centre of the dipole is given as
Work Done in Rotation
The concept of work done in rotation is central to understanding how energy is transferred in rotational systems, much like how work is done in linear motion. When a force is applied to an object, causing it to rotate around an axis, work is done to change the object's rotational state. This is similar to pushing a door to open it, where the force applied at the handle causes the door to rotate about its hinges. The amount of work done depends on the magnitude of the force, the distance from the axis of rotation (which is the lever arm), and the angle at which the force is applied.
Then work done by electric force for rotating a dipole through an angle
And So work done by an external force is
For example
Potential Energy of a Dipole Kept in an Electric Field
The potential energy of a dipole in an electric field is a crucial concept that describes how a dipole interacts with an external electric field. A dipole consists of two equal and opposite charges separated by a distance, creating a dipole moment. When this dipole is placed in an electric field, it experiences a torque that tends to align it with the direction of the field.
As
So change in the Potential Energy of a dipole when it is rotated through an angle
Assuming
we can write
Equilibrium of Dipole
The equilibrium of a dipole in an electric field is a condition where the dipole experiences no net torque, resulting in a stable or unstable configuration. A dipole consists of two equal and opposite charges separated by a distance, creating a dipole moment. When placed in an external electric field, the dipole experiences forces that tend to rotate it, aligning the dipole moment with the field.
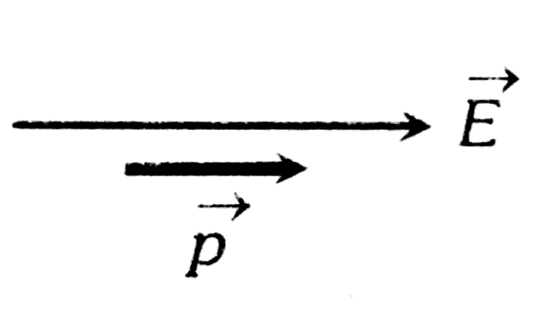
1. Stable Equilibrium
This occurs when the dipole is aligned with the electric field, meaning the dipole moment
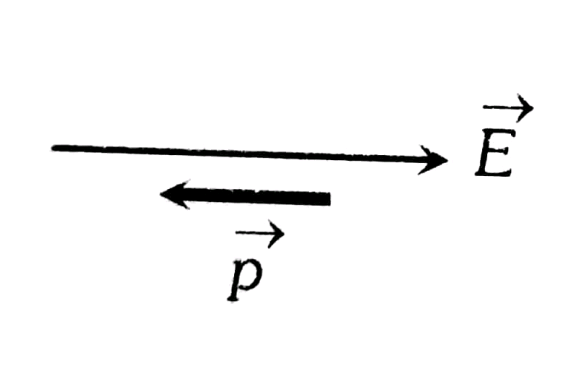
2. Unstable Equilibrium
This occurs when the dipole is aligned opposite to the electric field, meaning the dipole moment
Note
When
then
and it is important to note here that the dipole is not in equilibrium since

Recommended Topic Video
Solved Examples Based on Potential Energy of a Dipole In an Electric Field
Example 1: An electric dipole of moment
1) zero
2)
3)
4)
Solution:
Work done in rotation
Hence, the answer is the option (1).
Example 2: An electric field of 1000 V/m is applied to an electric dipole at an angle of 45o. The value of the electric dipole moment is 10-29 C.m. What is the potential energy of the electric dipole?
1)- 20 x 10-18 J
2)- 10 x 10-29 J
3) - 7 x 10-27 J
4)- 9 x 10-20 J
Solution:
Hence, the answer is the option (3).
Example 3: Two charges
1)
2)
3)
4)
Solution:
Stable Equilibrium
wherein

The potential energy of the electric dipole
Hence, the answer is the option (2).
Example 4: Electric charges
1) ql
2) 2 ql
3)
4) 4 ql
Solution:
Hence, the answer is the option (1).
Example 5: When an electric dipole
1)
2)
3)
4)
Solution:
Not in equilibrium
wherein
Maximum torque
Summary
The potential energy of a dipole in an electric field is a key concept in understanding how dipoles align with external fields, resulting in changes in their potential energy. This interaction leads to either stable or unstable equilibrium, depending on the dipole's orientation. The work done in rotating a dipole within an electric field further illustrates how systems move toward states of lower energy, a principle observed in both natural and engineered processes.

