Ammonification - Overview, Examples, Defination
Ammonification is a crucial step of the nitrogen cycle where decomposers convert organic nitrogen into ammonia (NH₃) or ammonium ions (NH₄⁺). It helps recycle nutrients, improve soil fertility, and support plant growth. Learn its process, role, and NEET exam relevance.
This Story also Contains
- What is Ammonification?
- Ammonification in the Nitrogen Cycle
- Process Of Ammonification
- Importance Of Ammonification for Soil Fertility
- Ammonification in Agriculture and Composting
- Ammonification vs Other Nitrogen Cycle Processes
- Weightage and Questions Types
- Ammonification NEET MCQs
- FAQs on Ammonification
- Recommended Video On Ammonification

Nitrogen is part of amino acids, proteins, and nucleic acids and is often a limiting nutrient in plants. Plants can use two inorganic forms of nitrogen: ammonium (NH₄⁺) and nitrate (NO3), and some organic forms, such as amino acids. The main reservoir of nitrogen is the atmosphere, which is 80% nitrogen gas (N2). The major pathway for nitrogen to enter an ecosystem is via nitrogen fixation. The conversion of N2 by bacteria to forms that can be used to synthesize nitrogenous organic compounds.
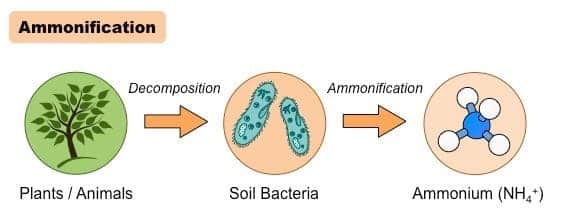
Ammonification is the stage of the nitrogen cycle in which decomposers like bacteria and fungi convert dead organic nitrogen into ammonia (NH3) or ammonium ions (NH4⁺). This process is vital in the nitrogen cycle because it helps ensure that nutrients are recycled back into a form available to plants, thereby maintaining mineral nutrition.
What is Ammonification?
Ammonification is the breakdown of organic matter by decomposers, primarily bacteria or fungi, inside dead plants or animals, and then converting it back into ammonia (NH3) or ammonium ions (NH₄⁺). The recycling of nitrogen in the ecosystem is thus again taken up by this process, as it ensures that nitrogen enters the soil in a form useful for the plants, and it will continue to make the soil healthier and fertile.
Ammonification can be simply defined as the breakdown of organic nitrogen into ammonia, which reintroduces the nitrogen in the soil for further absorption by plants.
Ammonification in the Nitrogen Cycle
The other critical step of the nitrogen cycle is ammonification. It is the process of movement of nitrogen between the atmosphere, soil, and organisms through biogeochemical cycles. The absence of ammonification would mean an inability to use the nitrogen trapped in organic matter for growing plants and, hence, breaking ecosystems.
Process Of Ammonification
The ammonification process begins with dead plant and animal matter, and excreted wastes. Here is the procedure divided into individual steps:
Step 1: Decomposition of Organic Nitrogen
When plants and animals die, decomposer organisms, mostly bacteria and fungi, feed on the dead organic matter.
These organisms metabolize nitrogen-containing compounds such as proteins, nucleic acids, and urea, whereby they release ammonia (NH₃) or ammonium ions (NH₄⁺).
Step 2: Conversion to Ammonia
Decomposer bacteria convert organic nitrogen compounds to ammonia. By undergoing enzymatic reactions, the chemical equation for ammonification is as follows:
Proteins + Water through enzyme activity → Amino acids
Amino acids through microbial action → Ammonia (NH₃) or Ammonium ions (NH₄⁺)
Step 3: Emission Of Ammonia
Once the ammonia is formed, it may stay as ammonium ions (NH₄⁺) in the soil, if the soil is acidic or it diffuses into the atmosphere as ammonia gas (NH₃).
Ammonification Reaction
A simple equation can be represented as:
Organic Nitrogen (Proteins/Dead Material) → Bacteria → Ammonia (NH₃) + Water (H2O).
Ammonium ions (NH₄⁺) are either absorbed directly or further processed through nitrification, converting it to nitrates (NO₃⁻), a readily available source of nitrogen for plants.
Importance Of Ammonification for Soil Fertility
It is crucial for the overall health and fertility of soil. It is involved in recycling nitrogen from dead organic matter that otherwise remains locked and unavailable for plant growth. It also keeps the nitrogen cycle running, a necessity to keep ecosystems balanced.
Ammonification in Agriculture and Composting
Ammonification replenishes the nitrogen source in soil, which is essential for plant growth and crop production improvement.
Organic fertilizers rely on the process of ammonification to replenish the nitrogen source in soil throughout various agricultural farms.
A good example of ammonification in action is in composting. Organic wastes such as decaying plant material, kitchen wastes, and manure from animals decompose with ammonification.
This converts the nitrogen of these materials into ammonia which can then be absorbed by the plants for growth improving soil fertility.
Ammonification vs Other Nitrogen Cycle Processes
Process | Description | Key Organisms |
Conversion of atmospheric nitrogen (N2) into ammonia (NH3) by nitrogen-fixing bacteria | Rhizobium, Azotobacter | |
Nitrification | Conversion of ammonia into nitrites (NO2-) and then into nitrates (NO3-) by nitrifying bacteria | Nitrosomonas, Nitrobacter |
Ammonification | Decomposition of organic matter into ammonia by decomposers | Decomposer bacteria, fungi |
Denitrification | Conversion of nitrates back into atmospheric nitrogen (N2) | Denitrifying bacteria |
Weightage and Questions Types
Exam Type | Weightage of Ammonification | Types of Questions |
CBSE Board Exams | 4-6% | Definitions, role in the nitrogen cycle, examples |
NEET | 1-2% | MCQs, assertion-reason questions, reaction-based questions |
Nursing Entrance Exams | 2-3% | True/false, scenario-based questions related to soil health |
Paramedical Entrance Exams | 1-2% | Case studies on soil nitrogen management |
Ammonification NEET MCQs
Q1. The correct sequence of stages in nitrogen fixation is
Ammonification, nitrification and denitrification
Nitrification, denitrification and ammonification
Denitrification, nitrification and ammonification
Ammonification, denitrification and nitrification
Correct answer: 1) Ammonification, nitrification and denitrification
Explanation:
The correct order of steps in nitrogen fixation is:
Nitrogen Fixation Nitrogen-fixing bacteria convert the atmospheric nitrogen to ammonia (NH₃).
Nitrification: Nitrifying bacteria break down ammonia in the following manner: oxidation into nitrites to nitrates.
Assimilation: Plants absorb nitrates and assimilate them into organic compounds.
Ammonification: Organic nitrogen is converted back into ammonia by decomposers.
Denitrification: Nitrates are reduced to nitrogen gas (N₂), returning it to the atmosphere.
Hence the correct answer is Option (1) Ammonification, nitrification and denitrification.
Q2. Decomposition of organic nitrogen of dead plants and animals into ammonia is called
Nitrogen fixation
Nitrification
Nitration
Ammonification
Correct answer: 4) Ammonification
Explanation:
The decomposition of organic matter containing nitrogen, such as decaying dead plants and animals, forms ammonia. In such a microbially-based process, decomposers break down proteins and nucleic acids that happen to contain nitrogen and consequently release ammonia in the soil. Released ammonia is taken by the plants or continues further conversion into nitrates during nitrification and serves as an integral part of the nitrogen cycle.
Hence, the correct answer is option 4) Ammonification.
Q3. Plants accumulate _________in the form of ammonium
Oxygen
Carbon
Nitrogen
All of the above
Correct answer: 3) Nitrogen
Explanation:
Plants accumulate nitrogen in the form of ammonium. The reduction of atmospheric nitrogen to ammonia is called nitrogen fixation. Higher plants generally utilize the oxidized forms such as nitrate (NO3-) and nitrite (NO2-) or the reduced form (N) of nitrogen, which is made available by a variety of nitrogen fixers. Nitrogen is a constituent of amino acids, proteins, hormones, chlorophyll, and many vitamins.
Hence, the correct answer is option 3) Nitrogen.
Also Read:
FAQs on Ammonification
Define ammonification.
Ammonification is the biological process in which organic nitrogen compounds present in dead plants, animals, and waste products are converted into ammonia (NH₃) or ammonium ions (NH₄⁺). This conversion is carried out by decomposer organisms. It is a crucial step of the nitrogen cycle because it transforms complex organic nitrogen into a simpler form that can re-enter soil nutrient pools. Without ammonification, nitrogen would remain locked in organic matter and unavailable to plants.
Which organisms carry out ammonification?
Ammonification is mainly carried out by decomposer microorganisms, especially bacteria and fungi. Examples include species of Bacillus, Pseudomonas, and fungi like Aspergillus and Penicillium. These organisms break down proteins, nucleic acids, and other nitrogenous wastes into ammonia. Thus, they act as recyclers in the ecosystem, ensuring a continuous supply of nitrogen for plant uptake.
What is the role of ammonification in the nitrogen cycle?
The role of ammonification is to recycle nitrogen locked in organic matter back into the ecosystem. By converting dead organic nitrogen into ammonia or ammonium ions, it replenishes the soil with nitrogen in a form that plants can directly absorb. This ensures soil fertility, supports plant growth, and maintains the balance of mineral nutrition. Without ammonification, nitrogen would accumulate in dead biomass and disrupt the nitrogen cycle.
Give an example of ammonification.
A common example of ammonification is the composting process. When plant residues, food scraps, or animal wastes are decomposed by microbes, the nitrogen present in proteins and other organic compounds is broken down and released as ammonia. This ammonia enriches the compost, making it a nitrogen-rich manure. Such natural recycling of nitrogen helps in sustainable agriculture and soil fertility management.
Recommended Video On Ammonification
Frequently Asked Questions (FAQs)
Nitrogen is the abundant gas in the atmosphere which makes78% of air.It is essential component for proteins,dna.It is required for growth,metabolic process and reproduction.Nitrogen is converted to ammonia and nitrates by nitrifying and ammonifying bacteria.So that plants can access it.
ammonification-It is the process of conversion of organic nitrogen(dead organisms,feces,urea) into ammonia by bacteria or fungi.they break down dead organisms.
Nitrification-Oxidation of ammonium ions into nitrate by nitrifying bacteria.nitrate is assimilated by plants.
Symbiotic bacteria(depending on other organisms) are found at the root nodules of plants and they take shelter from plants by providing nitrogen to plants through conversions.
Non symbiotic bacteria(not dependent on other organisms) live freely and they fix nitrogen for their own purpose.
Ammonification Definition:Ammonification is the process of converting organic nitrogen from dead organisms to ammonia by microorganisms.
Ammonification Importance
It provides essential nitrogen for plants that do not have a symbiotic relationship.
It maintains the ecosystem by recycling the dead organisms organic nitrogen compounds such as amino acids,dna,urea.
It balances the nitrogen in the atmosphere and the ecosystem .
It is crucial as it converts organic nitrogen into inorganic nitrogen ammonia.
