Pyruvate: Formula, Synthesis, Oxidation, Carboxylation: Definition, Structure & Uses
Pyruvate is the three-carbon end product of glycolysis and a central metabolic intermediate that connects carbohydrate, fat, and protein metabolism. It serves as the key branching point directing cells toward aerobic respiration (Acetyl-CoA) or anaerobic pathways (lactate or ethanol). Understanding pyruvate’s synthesis, oxidation, and carboxylation is essential for cellular respiration and NEET examination biology.
This Story also Contains
- What Is Pyruvate?
- Chemical Structure & Formula
- Synthesis of Pyruvate (Glycolysis End Product)
- Pyruvate as a Metabolic Crossroad
- Fate of Pyruvate (Aerobic vs Anaerobic)
- Regulation of Pyruvate Metabolism
- ATP Yield From Pyruvate Pathways
- Pyruvate NEET MCQs (With Answers & Explanations)
- Recommended Video On 'Pyruvate: Formula, Synthesis, Oxidation, Carboxylation'

What Is Pyruvate?
Pyruvate is regarded as one of the vital intermediates in quite several metabolic pathways. It is the end product of glycolysis, hence a critical element in cellular respiration. Pyruvate enters the cycle that occurs in glycolysis with the cycle of citric acid and oxidative phosphorylation.
Role in Glycolysis and Cellular Respiration
The glycolysis pathway changes one glucose into two pyruvates, generating two ATP molecules and two NADH molecules.
Pyruvate bears importance for cell respiration because it can further be metabolised for energy formation.
Relation to Metabolism
Pyruvate is a metabolic crossroad that can link carbohydrate, fat, and protein metabolic processes.
It can be converted into acetyl-CoA for the citric acid cycle or into lactic acid during anaerobic respiration.
Pyruvate vs Pyruvic Acid
Pyruvic acid is the form of the pyruvate in which it is in a protonated state (having added an H⁺).
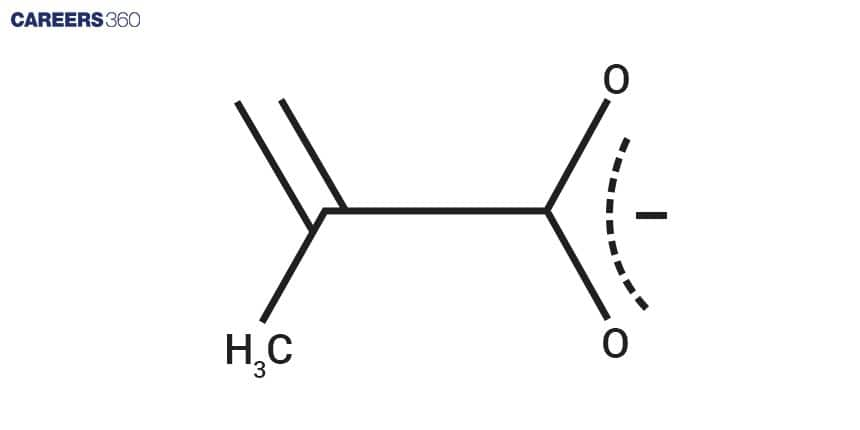
Chemical Structure & Formula
Chemical Structure and Molecular Formula:
Molecular formula: C₃H₄O₃
Chemical structure: CH₃COCOOH, (pyruvic acid), CH₃COCOO⁻(pyruvate)
Physical Properties
State: Solid at room temperature
Colour: Colorless to white
Melting Point: 165°C (329°F)
Solubility: Soluble in Water
Synthesis of Pyruvate (Glycolysis End Product)
The synthesis of pyruvate involves:
Steps Leading to Pyruvate Formation
Conversion to glyceraldehyde-3-phosphate and dihydroxyacetone phosphate.
Oxidation and phosphorylation to 1,3-bisphosphoglycerate.
Conversion to 3-phosphoglycerate, 2–2-phosphoglycerate and then phosphoenolpyruvate.
Final conversion to pyruvate.
Enzymes Involved
Hexokinase
Phosphofructokinase
Pyruvate kinase
Energy Yield
2 molecules of ATP (net gain) per molecule of glucose
2 NADH molecules
Pyruvate as a Metabolic Crossroad
The details are given below:
Link Reaction (Conversion to Acetyl-CoA)
Pyruvate is decarboxylated and attached to CoA to produce acetyl-CoA.
It gives NADH and CO₂.
Enzyme Complex: Pyruvate Dehydrogenase
A multi-enzyme complex made up of E1 (pyruvate dehydrogenase), E2 (dihydrolipoyl transacetylase), and E3 (dihydrolipoyl dehydrogenase).
It helps to convert pyruvate to acetyl-CoA.
Important in replenishing citric acid cycle intermediates (anaplerotic reactions) and gluconeogenesis.
Fate of Pyruvate (Aerobic vs Anaerobic)
The metabolic fate of pyruvate after glycolysis is:
Aerobic Pathway — Pyruvate Oxidation
In the presence of sufficient oxygen, pyruvate is transported into the mitochondria and then converted to acetyl-CoA by the pyruvate dehydrogenase complex. The produced acetyl-CoA will enter the citric acid cycle (Krebs cycle), where it becomes further oxidized into ATP, NADH, and FADH2 required for the electron transport chain.
Anaerobic Pathway — Reduction Reactions
In the absence of, or when oxygen is limited, the cells resort back to anaerobic pathways for energy generation.
In lactic acid fermentation, when the amount of oxygen is inadequate during exercise or when oxygen demand is high, pyruvate is converted to lactate.
Alcoholic fermentation in yeast and some bacteria, pyruvate is converted to ethanol and carbon dioxide through alcoholic fermentation.
Pyruvate Carboxylation
Biotin-dependent enzyme.
Catalyses the carboxylation of pyruvate to oxaloacetate.
Importance
Gluconeogenesis: synthesis of glucose from noncarbohydrate sources
Anaplerotic reactions: replenishing citric acid cycle intermediates
Regulation of Pyruvate Metabolism
The pyruvate metabolism is regulated by:
Allosteric Regulation
Activator is fructose-1,6-bisphosphate
Inhibitors is ATP, acetyl-CoA, NADH
Hormonal Regulation
Insulin stimulates glycolysis.
Glucagon stimulates gluconeogenesis.
Feedback Mechanisms
High levels of ATP inhibit glycolytic enzymes.
High levels of ADP activate glycolytic enzymes.
ATP Yield From Pyruvate Pathways
The details are given below:
Glycolysis
Net gain of 2 ATP molecules per glucose molecule.
2 NADH molecules per glucose molecule.
Citric Acid Cycle
Each acetyl-CoA produces 3 NADH, 1 FADH₂, and 1 GTP (equivalent to ATP).
Total ATP yield from the complete oxidation of one glucose molecule: 30 - 32 ATP.
Pyruvate NEET MCQs (With Answers & Explanations)
Important topics for NEET are:
Chemical formula of pyruvate
Regulation of Pyruvate metabolism
ATP yield from pyruvate
Practice Questions for NEET
Q1. In anaerobic respiration, pyruvic acid undergoes
Krebs cycle
Electron Transport Chain
Fermentation
Both a and b
Correct answer: 3) Fermentation
Explanation:
There are three major ways in which different cells handle pyruvic acid produced by glycolysis:
Lactic acid fermentation:- Under specific anaerobic conditions, particularly within animal muscle cells and certain bacterial species, pyruvic acid undergoes reduction to lactic acid facilitated by the enzyme lactate dehydrogenase, concurrently resulting in the oxidation of NADH to NAD+
Alcoholic fermentation:- In species such as yeast and various bacteria, the process of decarboxylation transforms pyruvic acid into acetaldehyde which results in ethanol eventually.
Aerobic respiration:- a cellular process in which glucose is completely oxidized to produce energy in the presence of oxygen.
Hence, the correct answer is option 3) Fermentation.
Q2. The key product of glycolysis is
Acteyl CoA
Phosphoenolpyruvate
Pyruvate
Carbon dioxide
Correct answer: 3) Pyruvate
Explanation:
The main byproduct of glycolysis is pyruvic acid. The pyruvate's metabolic requirement is contingent upon the cell's metabolic requirements. There are three major ways in which different cells handle pyruvic acid produced by glycolysis:
Lactic acid fermentation
Alcoholic fermentation
Aerobic respiration
Hence, the correct answer is an option 3) The key product of glycolysis is pyruvate.
Q3. After glycolysis, pyruvate can be converted into which of the following?
Acetyl CoA
Lactate
Ethanol
All of the above
Correct answer: 4) All of the above
Explanation:
In the absence of oxygen, pyruvate cannot enter the aerobic Krebs cycle, so it undergoes fermentation to generate ATP. In animal cells, pyruvate is converted into lactate by lactate dehydrogenase, while in yeast and some bacteria, pyruvate is converted into ethanol by the enzyme pyruvate decarboxylase. Alternatively, pyruvate can be converted into acetyl-CoA by the enzyme pyruvate dehydrogenase, which then enters the Krebs cycle for further ATP production.
Hence, the correct option is 4) All of the above.
Also Read:
Recommended Video On 'Pyruvate: Formula, Synthesis, Oxidation, Carboxylation'
Frequently Asked Questions (FAQs)
In other words, under anaerobic conditions, pyruvate is converted either into acetyl-CoA, which enters the citric acid cycle or into lactate during anaerobic respiration.
Pyruvate carboxylation is a process whereby pyruvate is converted into oxaloacetate. Such a process is crucial for gluconeogenesis and refilling the citric acid cycle.
Allosteric, hormonal, and negative feedback mechanisms control the pyruvate metabolism through some of the key enzymes and energy molecules such as ATP and ADP.
Pyruvate is the crucial intermediate that further connects glycolysis with the citric acid cycle and, thus, has a key role in garnering energy.
Pyruvate is synthesised via the pyruvate pathway, where glucose is broken down into the resulting product, which is pyruvate.
