Absorbance Vs Concentration: Introduction, Importance of Beer’s Law Equation Explained
There is a relationship between absorbance and concentration that is described by Beer-Lambert Law or Beer’s Law. Beer’s Law stated that in water the absorbance by light absorbing substances is directly proportional to its concentration and is expressed by the equation given as, A = εLc.
This Story also Contains
- Beer’s Law known as Lambert- Beer Law
- The Beer’s Law Equation
- How to use the Beer’s Law
- Importance of the Beer's Law
- Factors Affecting the Absorbance
- Various Names for the Beer-Lambert Law
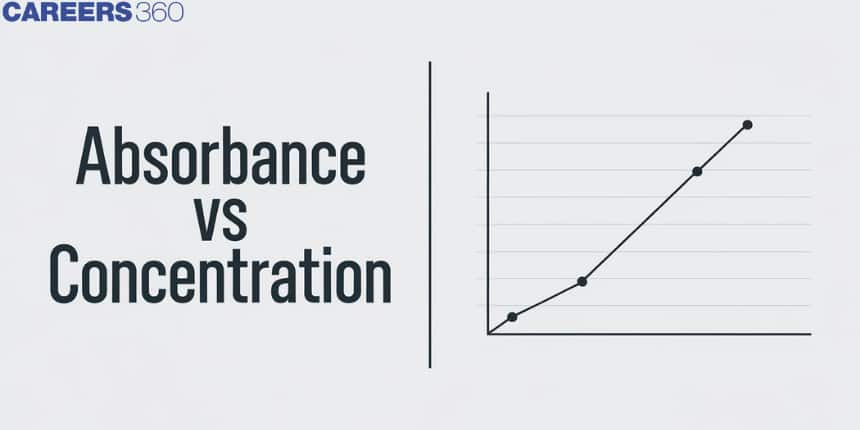
The solution of high concentration absorbs more light in comparison to light absorbed by the solution of low concentration. This law permits you to examine a phosphate concentration after examining the absorbance because the concentration and absorbance both are proportional to each other.
The absorbance of a solution is only affected by the concentration. Hence, as an example, when the concentration of the organic materials in water would be double then the UV absorbance (UVA) would also be double.
Beer’s Law known as Lambert- Beer Law
The Beer’s Law is a law that contains an equation that relates light attenuation to the substance qualities. According to Beer's law, the concentration of the solution is directly proportional to the absorbance of that solution. The concentration of a chemical in a solution is determined by using a colorimeter or spectrophotometer.
The Beer’s Law Equation
The Beer's law is expressed in the form of equation given as follows :
A = εLc
where,
A denotes the amount of the light that is absorbed by the sample at a particular wavelength.
ε denotes the coefficient of extinction at the molar level.
L denotes the time period of light for which it passes through the solution.
c denotes the value of the concentration of the species that absorbs light.
How to use the Beer’s Law
The Beer - Lambert law can be calculated by many of the current devices only by comparing a vacant cuvette to the sample. It is very easy to make a graph using standard solutions to establish the concentration of specimens. For the solutions that are diluted, the graph represents a straight line in it and as the relationship between the absorbance and the concentration of the solution.
Importance of the Beer's Law
Beer's Law is very important in the field of physics, chemistry and meteorology. In the field of chemistry, the Beer Law is used to find the concentration of chemicals, assess oxidation, and to monitor the deterioration of polymers. The abrasion of radiation by the atmosphere of the earth is similarly explained by this law. While this rule is generally associated with light, it also helps scientists in understanding the abrasion of beams of particles like neutrons.
Factors Affecting the Absorbance
The absorbance is affected by one factor that is the concentration (c ) of the sample. When the concentration of the solution increases then the radiation of light is more absorbed by the substance as a result of it, the absorbance of the solution increases. Hence, we can say that the concentration of the solution is directly proportional to its absorbance power.
The second factor affecting the absorbance is the length of the path (L). If the length of the path becomes longer then the molecules of the radiation beam in the path increases as a result of it the absorbance power of the solution increases. So we can say that the path length is directly proportional to the absorbance.
The third factor that affects the absorbance of the solution is the molar absorptivity (ε), if the value of concentration is given in moles/litre and the length of the path is given in centimeters. In some other studies, this is generally known as the extinction coefficient.
Hence, the Beer - Lambert Law has been made to show the relationship between these factors as the concentration of the solution, length of the path, and molar absorptivity are all directly proportional to absorbance power of the solution.
Various Names for the Beer-Lambert Law
The Beer law is also known by various names such as the Lambert - Beer Law, the Beer - Lambert and the Beer - Lambert - Bouguer Law. Beer's law is known by so many names because it contains multiple laws.
In the year 1729, the great scientist Pierre Bouguer discovered this law.
Later, in the year 1760, scientist Johann Heinrich Lambert resolved Bouger’s discovered law and said that the absorbance power of a sample is directly proportional to the length of the path of light. Although Lambert did not declare this discovery yet he got the credit for it.
In the year 1852, another great scientist named August Beer discovered a law which points to the relation that the absorbance power of solution is directly proportional to the concentration of the solution.
Frequently Asked Questions (FAQs)
The Spectrometers and Colorimeters are the special devices that are used by the scientists to measure the absorbance of a solution.
The range of absorbance that is useful for colorimeters is from 0.1 to 1. The value of absorbance that is greater than or equal to 1 is not useful and above this value the Beer's law failed.
The term ε denotes the coefficient of extinction at the molar level of the solution, in the equation of the Beer - Lambert Law.
The radiation of Solar light or stellar radiation in the atmosphere can be explained by using the Beer - Lambert Law.
The Beer - Lambert Law was discovered by the great scientist Pierre Bouger in the year 1729. He was the French mathematician who gave expression to this law.
