Catalysis
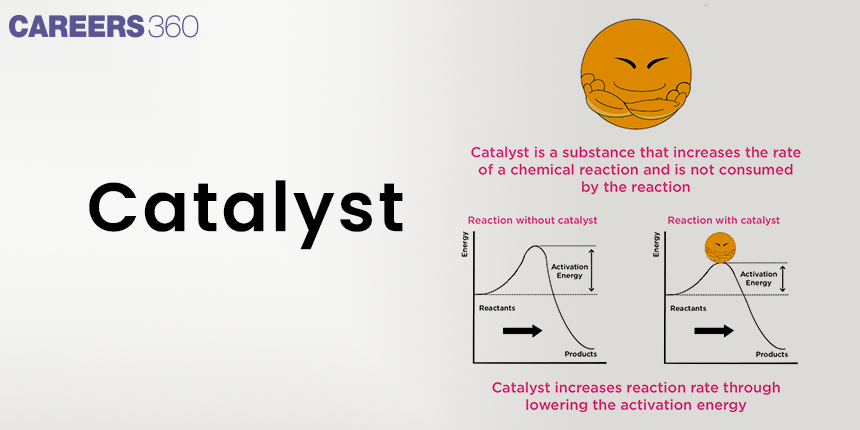
Interestingly enough, catalysis is considered to be one extremely captivating and very topically important concept of chemistry since it has a great influence on our everyday lives and on many industrial processes. For instance, just think of the frustration that would be in case one needs to wait for some hours for an automobile catalytic converter to cause full neutralization of toxic gases or in case industrial chemical reactions need days instead of minutes. All these difficulties are solved by catalysts, which are substances able to speed up chemical reactions without their consumption.
This Story also Contains
- Homogeneous and Heterogeneous Catalysis
- Promoters and Poisons
- Shape Selective Catalysis
- Surface Roughening of Zeolites
- Some Solved Examples
- Conclusion
Homogeneous and Heterogeneous Catalysis
Homogeneous Catalysis
The reaction is homogeneous if the phase of the catalyst is at the same phase as that of the reactants. Usually, the phase is a liquid solution, in this case, the esterification is produced by reacting carboxylic acids and alcohols under acid catalysis.
Heterogeneous Catalysis
In heterogeneous catalysis, the catalysts act in a phase that is different from that of the reactants. It normally involves solids with gaseous or liquid reactants, for example, platinum in catalytic converters for reducing auto emissions.
Promoters and Poisons
Promoters: These are substances that, when added to a catalyst, enhance its activity. The addition of minute quantities of, for example, molybdenum to catalysts for the Haber process, iron, increases greatly ammonia production.

Poisons
Catalyst Poison: A catalyst poison is a substance that depresses or hinders the catalytic activity. For example, sulfur compounds act as poisons for some metal catalysts, as these cake the main active sites of the catalyst and thus lower its efficiency.
Some of the unique properties of solid catalysts are that they may be porous with a high area and contain some active sites that may catalyze reactions very effectively. Because of this reason, these catalysts are very vital in many applications—for example—in the cracking of petroleum hydrocarbons using zeolites as catalysts.

Shape Selective Catalysis
This way, a catalyst can afford reaction selectivity depending on size and shape of reactant molecules. One of its examples comes from a catalyst used in shaping selective catalysis that takes place during hydrocarbon processing. Zeolites, used for converting low-octane feedstock into gasoline, are a nice example of this.
Surface Roughening of Zeolites
Enzymes are biological catalysts and drive nearly all of the chemical reactions that power life. They are highly selective and work at a rapid pace in even the mildest conditions. Enzymes are able to digest food, respire within cells, or catalyze any other reaction just as important, by putting these minute powers of nature into play.

Recommended topic video on(Catalysis)
Some Solved Examples
Q.1Which of the following is not an example of a heterogeneous catalyst?
1)Haber's process of NH3 synthesis
2)Catalytic conversion of SO2 to SO3 in contact process
3)Oxidation of ammonia into nitric oxide in Ostwald’s process.
4) (correct)Acidic hydrolysis of methyl acetate
Solution:
In the hydrolysis of esters, all reactants, products, as well as Catalysts (H+), are in the aqueous phase and hence it is an example of Homogenous catalysis in Haber's process, the catalyst is Fe(s)In the Contact process, the catalyst is V2O5(s)In Ostwald's process, the catalyst is Pt(s)In all the above reactions, the reactant(s) and product(s) are gaseous.
Hence, the answer is the option (4).
Example 2: In homogeneous catalyst reactions, the rate of reactions:
1. Depends upon the concentration of the catalyst(correct)
2. Independent of the concentration of the catalyst
3. Depends upon the free energy change
4. Depends upon the physical state of the catalyst
Solution:
In homogeneous catalysis, the catalyst is in the same phase as that of the reactants, so its concentration will affect the rate of reaction.
Hence, the answer is option (1).
Example 3: Which of the following is not an example of a heterogeneous catalytic reaction?
1. Ostwald's process
2. Combustion of coal(correct)
3. Hydrogenation of vegetable oils
4. Haber's process
Solution:
Heterogeneous Catalysis: The catalytic process in which the reactants and catalyst are in different phases is known as heterogeneous catalysis.
As we have learned in combustion, we do not require any catalysts for the combustion of coal. So, there will be no sense of a heterogeneous catalytic reaction.
Hence, the answer is option (2).
Conclusion
Homogeneous and heterogeneous catalysis, promoters, and poisons, solid catalysts characteristics, shape-selective catalysis, and enzyme catalysis, all discussed within the article, broadly encompass catalysis. From all these concepts taken for review in this paper, we could observe how the catalysts are meant to accelerate chemical reactions, producing great effects on both industrial processes and, for the benefit of the latter, on the environment and biological systems. Of course, the extracted relevance toward the real-life application of catalysis underpins the relevance of the advancement of science and technology.
Frequently Asked Questions (FAQs)
Enzymes are used as biological catalysts to speed up biochemical reactions with very high sensitivity and efficiency at levels that can be termed mild.
The catalysts used in homogeneous catalysis exist in the same phase as the reactants whereas heterogeneous catalysis uses catalysts that exist in a phase different from the reactant.
Promoters increase work activity in catalysts, but they reduce the catalytic activity or even nullify it in poisons.
Solid catalysts usually have high surface areas, porosities, and particular special active sites encouraging chemical reactions.
