Balancing Chemical Equations - Definition, Method, Formulas, Examples, FAQs
Have you ever wondered why the number of atoms on both sides of a chemical equation must be the same? What would happen if we didn’t balance an equation? Would it break the laws of nature? We will get these answers after reading this article on balancing a chemical equation. When the reactant and product sides of an equation have the same number of each element, the equation is balanced
This Story also Contains
- Chemical Equation
- Stoichiometric Coefficient
- Balancing Of Chemical Equation Method
- Some Solved Examples
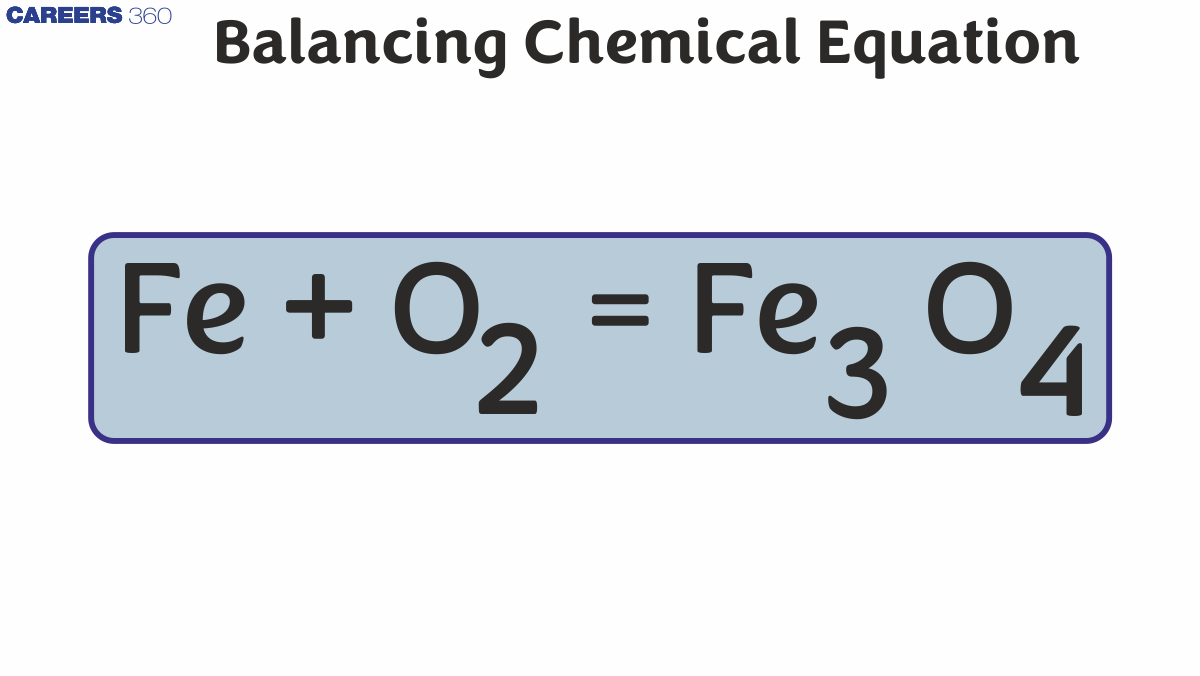
To accurately depict the rule of conservation of mass, equations must be balanced. The inclusion of Stoichiometric coefficients for the reactants and products is required to balance chemical equations. This is crucial because a chemical equation must follow the laws of conservation of mass and constant proportions,
Chemical Equation
A chemical equation is a symbol for a chemical reaction in which the reactants and products are represented by their chemical formulae.
$2 \mathrm{H}_2+\mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}$ is an example of a chemical equation that depicts the reaction between Hydrogen and oxygen to generate water.
The part of the chemical equation to the left of the ‘→' sign is the reactant side, while the part to the right of the arrow symbol is the product side.
Stoichiometric Coefficient
The total number of molecules of a chemical species that participate in a chemical reaction is described by a stoichiometric coefficient.
It calculates the ratio between the responding species and the reaction products.
The stoichiometric coefficients of O2 and H2O in the reaction $\mathrm{CH}_4+2 \mathrm{O}_2 \rightarrow \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}$ are 2 and 1, respectively, in the equation $\mathrm{CH}_4+2 \mathrm{O}_2 \rightarrow \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}$.
Stoichiometric coefficients are assigned in a way that balances the total number of atoms of an element on the reactant and product sides when balancing chemical equations.
|
Related Topics |
Balancing Of Chemical Equation Method
When it comes to balancing chemical equations, the first step is to acquire the entire imbalanced equation. The combustion process between propane and oxygen is used as an example to demonstrate this method.
Step 1: Create an unbalanced equation using the chemical formulae of the reactants and products (if it is not already provided).
The molecular formula for propane is C3H8. It creates carbon dioxide (CO2) and water when it comes into touch with oxygen (O2) (H2O).
$\mathrm{C}_3 \mathrm{H}_8+\mathrm{O}_2 \rightarrow 3 \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}$
Step 2: On the reactant and product sides, compare the total number of atoms of each element. In this situation, the number of atoms on each side can be tabulated as follows.
|
Chemical equation: $\mathrm{C}_3 \mathrm{H}_8+\mathrm{O}_2 \rightarrow 3 \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}$ | |
|
Reactant side |
Product side |
|
3 C from $\mathrm{C}_3 \mathrm{H}_8$ |
1 C from CO2 |
|
8 H From $\mathrm{C}_3 \mathrm{H}_8$ |
2 H from H2O |
|
2 O from O2 |
3 O, 2 from CO2 and 1 from H2O |
Step 3: On the reactant and product sides, stoichiometric factors are now applied to compounds containing an element with a specific number of atoms.
The coefficient must balance the number of atoms on each side.
The hydrogen and oxygen atoms' stoichiometric coefficients are normally assigned last.
It's time to adjust the number of atoms in the reactant and product elements.
It's important to note that the total number of atoms of an element in one molecule of a species is calculated by multiplying the stoichiometric coefficient by the total number of atoms of that element in that molecule.
When the CO2 molecule is assigned the coefficient 3, the total number of oxygen atoms in CO2 becomes 6. The coefficient is first assigned to in this case.
|
Chemical equation: $\mathrm{C}_3 \mathrm{H}_8+\mathrm{O}_2 \rightarrow 3 \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}$ | |
|
Reactant side |
Product side |
|
3 Carbon atoms from C3H8 |
3 Carbon atoms from CO2 |
|
8 Hydrogen atoms from C3H8 |
2 Hydrogen atoms from H2O |
|
2 Oxygen atoms from O2 |
7 Oxygen atoms, 6 from CO2 and 1 from H2O |
Step 4: Step 3 is repeated until the reactant and product sides of the reactive elements have the same number of atoms. In this scenario, hydrogen is balanced next. The chemical equation is altered in this manner.
|
Chemical equation: $\mathrm{C}_3 \mathrm{H}_8+\mathrm{O}_2 \rightarrow 3 \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}$ | |
|
Reactant side |
Product side |
|
3 C from C3H8 |
3 C from CO2 |
|
8 H from C3H8 |
8 H from H2O |
|
2 O from O2 |
10 O, 6 from CO2, and 4 from H2O |
Now that the hydrogen atoms are balanced, the next element to be balanced is oxygen. There are 10 oxygen atoms on the product side, implying that the reactant side must also contain 10 oxygen atoms.
Each O2 molecule contains 2 oxygen atoms. Therefore, the stoichiometric coefficient that must be assigned to the O2 molecule is 5. The updated chemical equation is tabulated below.
|
Chemical equation: $\mathrm{C}_3 \mathrm{H}_8+5 \mathrm{O}_2 \rightarrow 3 \mathrm{CO}_2+4 \mathrm{H}_2 \mathrm{O}$ | |
|
Reactant side |
Product side |
|
3 Carbon atoms from C3H8 |
3 Carbon atom from CO2 |
|
8 Hydrogen atoms from C3H8 |
8 Hydrogen atoms from H2O |
|
10 Oxygen atoms from O2 |
10 Oxygen atoms, 6 from CO2 and 4 from H2O |
Step 5: After all of the individual elements have been balanced, the total number of atoms in each element on the reactant and product sides is compared once more.
The chemical equation is said to be balanced if there are no inequalities.
Every element now has the same number of atoms on the reactant and product sides in this example.
As a result, $\mathrm{C}_3 \mathrm{H}_8+5 \mathrm{O}_2 \rightarrow 3 \mathrm{CO}_2+4 \mathrm{H}_2 \mathrm{O}$ is the balanced chemical equation.
EXAMPLE:
$\mathrm{Al}+\mathrm{O}_2 \rightarrow \mathrm{Al}_2 \mathrm{O}_3$ is an unbalanced chemical equation.
Traditional Method
The reaction can be balanced using the old method as follows:
|
Chemical equation: $\mathrm{Al}+\mathrm{O}_2 \rightarrow \mathrm{Al}_2 \mathrm{O}_3$ | |
|
Reactant side |
Product side |
|
1 Aluminium atom |
2 Aluminum atoms |
|
2 oxygen atoms |
3 oxygen atoms |
The aluminium atoms are balanced first.
$\mathrm{Al}+\mathrm{O}_2 \rightarrow \mathrm{Al}_2 \mathrm{O}_3$
There are two oxygen atoms on the reactant side and three on the product side, so the oxygen atoms must be balanced now. As a result, there must be three O2 molecules that produce two Al2O3 atoms.
$2 \mathrm{Al}+3 \mathrm{O}_2 \rightarrow 2 \mathrm{Al}_2 \mathrm{O}_3$ is the chemical conversion equation.
Because the number of aluminium atoms on the product side has doubled, the number on the reactant side must have doubled as well
|
Chemical equation: $4 \mathrm{Al}+3 \mathrm{O}_2 \rightarrow 2 \mathrm{Al}_2 \mathrm{O}_3$ | |
|
Reactant side |
Product side |
|
4 Aluminium atoms |
4 Aluminum atoms |
|
6 oxygen atoms |
6 oxygen atoms |
Balanced chemical equation is found to be $2 \mathrm{Al}+3 \mathrm{O}_2 \rightarrow 2 \mathrm{Al}_2 \mathrm{O}_3$
Also check-
Some Solved Examples
Question.1 The number of moles of Br2 produced when two moles of potassium permanganate are treated with excess potassium bromide in an aqueous acid medium is:
1) 1
2) 3
3) 2
4) 4
Solution:
$
\mathrm{MnO}_4^{-}+\mathrm{Br}^{-} \rightarrow \mathrm{Br}_2+\mathrm{Mn}^{2+}
$
Oxidation half
$
\left[2 \mathrm{Br}^{-} \rightarrow \mathrm{Br}_2+2 e^{-}\right] \times 5
$
Reduction half
$
\left[\mathrm{SH}^{+}+\mathrm{MnO}_4^{-}+\mathrm{Se}^{-} \rightarrow \mathrm{Mn}^{+2}+4 \mathrm{H}_2 \mathrm{O}\right] \times 2
$
Adding both half reactions
$
\begin{gathered}
2 \mathrm{MnO}_4^{-}+10 \mathrm{Br}^{-}+16 \mathrm{H}^{+} \rightarrow 5 \mathrm{Br}_2+2 \mathrm{Mn}^{2+}+\mathrm{SH}_2 \mathrm{O} \\
2
\end{gathered}
$
2 moles of $\mathrm{MnO}_4^{-}$will produce 5 moles of $\mathrm{Br}_2$
Hence answer is 5 .
Hence, the answer is option (2).
Question.2 In button cells widely used in watches, number of correct statements from the followings:
a) Redox reaction is observed.
b) 2 molesof electrons are exchanged for 1 mole of $Zn$ metal
c) Ag is converted to $\mathrm{Ag}_2 \mathrm{O}$
d) $Z n$ is converted to $\mathrm{Zn}^{2+}$
Solution:
$
\mathrm{Zn}(s)+\mathrm{Ag}_2 \mathrm{O}(s)+\mathrm{H}_2 \mathrm{O}(l) \longrightarrow \mathrm{Zn}^{2+}+2 \mathrm{Ag}(\mathrm{~s})+2 \mathrm{OH}^{-}
$
2 mole $e^{-}$are utilized.
Redox reaction is observed.
2 molesof electrons are exchanged for 1 mole of $Zn$ metal
$Z n$ is converted to $\mathrm{Zn}^{2+}$
Hence, the answer is (3).
Question.3 What is the number of electrons used in the reduction of 1mol of nitrate in the presence of acid?
1) 2
2) 5
3) 10
4) 9
Solution:
$
2 \mathrm{NO}^{-3}+10 \mathrm{e}^{-}+12 \mathrm{H}^{+} \rightarrow \mathrm{N}_2+6 \mathrm{H}_2 \mathrm{O}
$
Hence, the answer is option (3).
Question 4: Balance the following reaction using the half-reaction method.
$\mathrm{I}_2+\mathrm{OH}^{-} \rightarrow x \mathrm{IO}_3^{-}+y \mathrm{I}^{-}+z \mathrm{H}_2 \mathrm{O}$
and find the value of $x+y+z$.
1) 8
2) (correct) 9
3) 10
4) 11
Solution:
Half-reactions-
1) $\mathrm{I}_2+\mathrm{OH}^{-} \rightarrow \mathrm{IO}_3^{-}$
2) $\mathrm{I}_2+12 \mathrm{OH}^{-} \rightarrow 2 \mathrm{IO}_3^{-}+6 \mathrm{H}_2 \mathrm{O}$
3) $\mathrm{I}_2+12 \mathrm{OH}^{-} \rightarrow 2 \mathrm{IO}_3^{-}+6 \mathrm{H}_2 \mathrm{O}+10 e^{-}$
Balance the equation by multiplying the second equation by 5 and adding we get;
$\begin{aligned}
& I_2+12 \mathrm{OH}^{-}+10 e^{-}+5 I_2 \rightarrow 10 I^{-}+2 I_3^{-}+6 H_2 O+10 e^{-} \\
& 3 I_2+6 O H^{-} \rightarrow 5 I^{-}+3 H_2 O+I O_3^{-} \\
& x=1, y=5, z=3 \quad x+y+z=9
\end{aligned}$
Hence, the answer is option (2).
Question 5: Which of the following is/are correct
1) (correct)$ 3 \mathrm{Cl}_2+6 \mathrm{NaOH} \rightarrow \mathrm{NaClO}_3+5 \mathrm{NaCl}+3 \mathrm{H}_2 \mathrm{O}$
2) (correct) $2 \mathrm{Cl}_2+2 \mathrm{Ca}(\mathrm{OH})_2 \rightarrow \mathrm{CaCl}_2+\mathrm{Ca}(\mathrm{OCl})_2+2 \mathrm{H}_2 \mathrm{O}$
3) $10 \mathrm{I}^{-}+2 \mathrm{MnO}_4^{-}+8 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{Mn}^{2+}+5 \mathrm{I}_2+16 \mathrm{OH}^{-}$
4) (correct) $\mathrm{XeF}_6+3 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{XeO}_3+6 \mathrm{HF}$
Solution:
(A) A is correct because $\mathrm{Cl}_2$ disproportionate into $\mathrm{ClO}_3^{-}+\mathrm{Cl}^{-}$
(B) is correct, it is bleaching powder formation method
(C) $\mathrm{I}^{-}+\mathrm{KMnO}_4 \xrightarrow{\mathrm{OH}^{-}} \mathrm{IO}_3^{-}+\mathrm{Mn}^{2+}$
(D) $\mathrm{XeF}_6+3 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{XeO}_3+6 \mathrm{HF}$
Hence, the answer is the options $(1,2,4)$.
Practice More Questions With The Link Given Below
| Balancing of Redox Reaction: Ion Electrode Method practice question and MCQs |
| Balancing of Redox Reaction: Oxidation Number Method practice question and MCQs |
Frequently Asked Questions (FAQs)
Balancing chemical equations is necessary to accurately represent chemical reactions. It also allows us to determine the stoichiometric relationships between the reactants and products, which can be useful for calculating the amount of reactants needed or the amount of products formed in a reaction.
The stoichiometric coefficients in a balanced chemical equation are determined by balancing the equation and ensuring that the number of atoms of each element is the same on both sides. The coefficients represent the ratios of the reactants and products in the reaction, so they must be in the lowest whole numbers possible.
A balanced chemical equation is one in which the number of atoms of each element in the reactants equals the number of atoms of that element in the products. This is necessary for the law of conservation of mass to be satisfied.
Ca(OH)2 + 2HNO3 → Ca(NO3)2 + 2H2O is the balanced chemical equation of the calcium hydroxide-nitric acid reaction.
The number of molecules in a chemical species which participate in a chemical reaction is called its stoichiometric coefficient.
It calculates the ratio between the either sides of the chemical reaction.
C3H8 + 5O2 → 3CO2 + 4H2O
