Laws of Chemical Combination for Elements and Compounds - Meaning, Definition, Examples, FAQs
One of the most important aspects of the subject of Chemistry is the study of chemical reaction. These chemical reactions take place according to certain laws, called the 'laws of chemical combination'. There are five basic Law of Chemical combination of Elements and Compounds of chemical composition that control chemical composition.
This Story also Contains
- The laws of chemical combination
- Some Solved Examples
- Practice More Questions From the Link Given Below:
- Summary
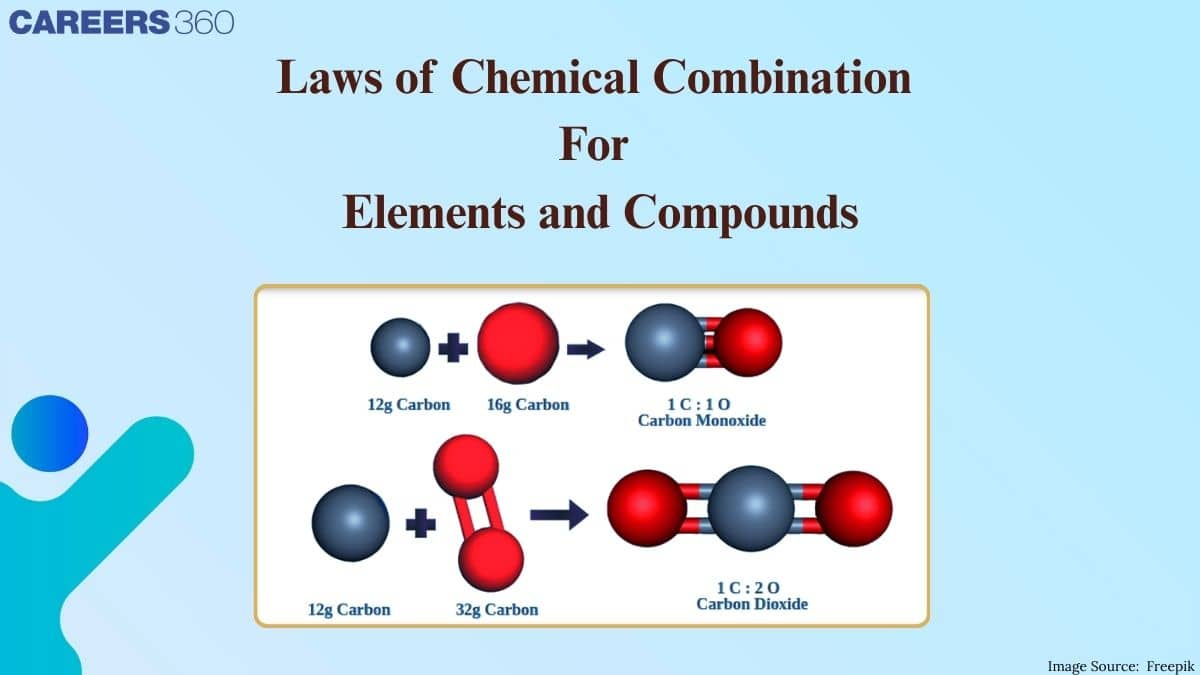
The laws of chemical combination
Whenever substances react, they do so according to certain laws. These laws are called the laws of chemical combinations. These basic laws are five in number and formed the basis of Dalton's Atomic Theory.
Law of Conservation of Mass :
It states that Matter can neither be created nor destroyed. This law formed the basis for several later developments in chemistry. In fact, this was the result of the exact measurement of masses of reactants and products, and carefully planned experiments performed by Lavoisier.
Law of Definite Proportions :
This law was given by, a French chemist, Joseph Proust. He stated that a given compound always contains exactly the same proportion of elements by weight.
Proust wprked with two samples of cupric carbonate - one of which was of natural origin and theother was synthetic one. He found that the composition of elements present in it was same for both the samples as shown below:
| % of Copper | % of Oxygen | % of Carbon | |
| Natural sample | 51.35 | 9.74 | 38.91 |
| Synthetic sample | 51.35 | 9.74 | 38.91 |
Thus, irrespective of the source, a chemical compound always contains the elements in fixed ratio by mass.
Law of Multiple Proportions :
This law was proposed by Dalton in 1803. According to this law, if two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element, are in the ratio of small whole numbers.
For example, carbon and oxygen combines with each other to form carbon monoxide (CO) and carbon dioxide (CO2).
In CO : 12 parts by mass of carbon combine with 16 part by mass of oxygen.
In CO2 : 12 parts by mass of carbon combine with 32 parts by mass of oxygen.
Ratio of masses of oxygen which combine with fixed mass of carbon in these compounds is 16:32 or 1:2, which is a simple whole number ratio.
Gay Lussac’s Law of Gaseous Volumes :
This law was given by Gay Lussac in 1808. He observed that when gases combine or are produced in a chemical reaction they do so in a simple ratio by volume provided all gases are at the same temperature and pressure.
This law may be illustrated by the following example:
It has been found that one volume of nitrogen combines with three volumes of hydrogen to form two volumes of ammonia gas.
$\mathrm{N}_2+3 \mathrm{H}_2 \rightleftharpoons 2 \mathrm{NH}_3$
Therefore, ratio by volumes of nitrogen, hydrogen and ammonia is 1:3:2, which is a simple whole number ratio.
Avogadro Law :
- According to this law, "Under similar conditions of temperature and pressure, the equal volume of gases the equal number of molecules. "
It means 10 ml of H2, O2, N2 or a mixture of gases have the same number of molecules.
It is used in:
(i) The deriving molecular formula of a gas
(ii) Determining atomicity of a gas
(iii) Deriving a relation
molecular mass = 2 x vapour density
M=2 X VD
(iv) Deriving the gram molecular volume
- Avogadro number (No or NA) = 6.023 x 1023
- Avogadro number of gas molecules occupy 22.4 litre or 22400 ml or cm3 volume at STP
- The number of molecules in 1 cm3 of a gas at STP is equal to Loschmidt number, that is, 2.68 x 1019
- The reciprocal of Avogadro number is known as Avogram.
Related Topics,
- Law Constant Proportion
- Balancing Chemical Equations
- Mole Concept Basics
- Difference Between Physical and Chemical Change
- Difference Between Compound and Mixture
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
Recommended topic video on (Laws of Chemical Combination for elements)
Also read -
Some Solved Examples
Example.1
The ratio of masses of oxygen and nitrogen in a particular gaseous mixture is 1:4. The ratio of a number of their molecule is :
1) 1 : 4
2) 7 : 32
3) 1 : 8
4) 3 : 16
Solution
We know
Mole = mass/molar mass
Avogadro's number is defined as the number of elementary particles (molecules, atoms, compounds, etc.) per mole of a substance. It is equal to 6.022×1023 mol-1 and is expressed as the symbol NA.
Now let the ratio be m, therefore, the mass of O2 is m and the mass of N2 is 4m.
Therefore, moles of O2 = m/32 and moles of N2 = 4m/28
Thus, $\frac{\text { molecules }_{\mathrm{O}_2}}{\mathrm{molecules}_{\mathrm{N}_2}}=\frac{\frac{\mathrm{m}}{32} \times \mathrm{N}_{\mathrm{A}}}{\frac{4 \mathrm{~m}}{28} \times \mathrm{N}_{\mathrm{A}}}=\frac{7}{32}$
Hence, the answer is the option (2).
Example. 2
What is the ratio of masses of oxygen that combine with a fixed mass of Hydrogen in water and Hydrogen Peroxide?
1) 1 : 2
2) 2 : 1
3) 2 : 3
4) 3 : 2
Solution
As we learnt in
$\mathrm{H}_2+\frac{1}{2} \mathrm{O}_2 \rightarrow \mathrm{H}_2 \mathrm{O}$
2g 16g 18g
$\mathrm{H}_2+\mathrm{O}_2 \rightarrow \mathrm{H}_2 \mathrm{O}_2$
2g 32g 34g
One mole of water has 2g hydrogen & 16g oxygen.
Similarly, one mole of hydrogen peroxide has 2g of hydrogen and 32 g of oxygen
Therefore, masses of oxygen combined with a fixed mass of hydrogen in water and hydrogen peroxide have a ratio of 16:32 = 1:2
Hence, the answer is the option (1).
Example .3
What is the ratio of the masses of oxygen that combine with a fixed value of nitrogen in the compounds nitrogen monoxide and nitrogen dioxide?
1) 2 : 3
2) 2 : 1
3) 3 : 2
4) 1 : 2
Solution
Let's take NO first, the chemical reaction follows:
$N_2+O_2 \rightarrow N O$
$\frac{\text { Mass of } N}{\text { mass of } O}=\frac{14}{16}=1: 1.143$
in NO2,
$2 \mathrm{NO}+\mathrm{O}_2 \rightarrow 2 \mathrm{NO}_2$
there are 2 N and 4 O in the reaction,
$\frac{\text { Mass of } N}{\text { mass of } O}=\frac{28}{64}=1: 2.286$
$\therefore \frac{\text { Mass of } \mathrm{O} \text { in } \mathrm{NO}}{\text { mass of } \mathrm{O} \text { in } \mathrm{NO}_2}=\frac{1.143}{2.286}=1: 2$
Hence, the answer is the option (4).
Practice More Questions From the Link Given Below:
NCERT Chemistry Notes:
Summary
The laws of chemical combination expresses how elements form compounds and directed our understanding of chemical reactions. The conservation of mass make sure that reactant mass equals product mass in a closed system. The law of definite proportions states that a compound always contains the same elements in fixed mass ratios, no matter how or where it's made. The law of multiple proportions shows that when two elements form different compounds, the ratios of one element combining with a fixed mass of the other are simple whole numbers ratio. These principles form the foundation of stoichiometry and expected chemistry.
Frequently Asked Questions (FAQs)
Dalton's atomic theory could explain the Law of Chemical combination of Elements and Compounds of chemical reactions.
The law of certain measurements, also known as the law of consistency, states that the elements that make up a chemical compound are always present at a fixed rate (according to their size). This measure does not depend on the source of the chemical element or the way it is prepared.
The ratio of substances to non-stoichiometric chemicals varies from sample to sample. Therefore, these compounds are different from the law of fixed size. Samples of objects that differ in their isotopic composition may also contravene the law of specified size because the two isotopic masses are distinctly different material. Natural polymers are also known for not always obeying the equality law.
The law of precise size was first issued by the French chemist Joseph Louis Proust in 1779. This is why this law is also known as Proust's law. The first compliance with this rule was first performed by French chemists Antoine Lavoisier and Joseph Priestley.
Water molecules comprise a mixture of hydrogen and oxygen atoms in a ratio of 2: 1. As they exist in a fixed size, water molecules adhere to the law of equality. Another example of a chemical compound that complies with the law of regular measurement is methane. To form a single methane molecule, 4 hydrogen atoms combine with one carbon atom.
Although this law is easily understood today, it was widely used in the late 18th century when chemical compounds did not have a proper meaning. The law of precise magnitude also contributed to the development of Dalton's atomic theory.
No, for all sorts of things, the law of some measure does not apply. Materials with a stable isotope compound tend to form a non-stoichiometric product. The role of certain elements in the crystal structure is replaced by their isotopes that make the crystalline structure different.
Atomic disintegration has been proven wrong: it is possible to further divide an atom into protons, neutrons, and electrons. However, the smallest particle that occurs in chemical reactions is the electron. Atoms of the same product are the same in every way, according to Dalton.
Antonine L.Lavoisier gave the Law of Chemical combination of Elements and Compounds.
