Cannizzaro Reaction Mechanism - Overview, Steps, Scope, Uses & Applications, FAQs
Have you ever wondered what happens when an aldehyde without an α-hydrogen is treated with a strong base? Such aldehydes undergo a special self-oxidation and self-reduction process known as the Cannizzaro reaction. The Cannizzaro reaction is a disproportionation process in which two molecules of an aldehyde combine with a hydroxide base to generate a primary alcohol and a carboxylic acid. This is exemplified in the example of benzaldehyde, which creates benzyl alcohol to benzoic acid. And disproportion is a redox reaction in which a mid-oxidation chemical transforms into two distinct compounds, one high and the other low.
This Story also Contains
- Cannizzaro Reaction Mechanism
- Mechanism of Cannizzaro Reaction
- Crossed reaction of Cannizzaro
- Scope of Cannizzaro reaction:
- Cannizzaro Reaction Application
- Some Solved Examples
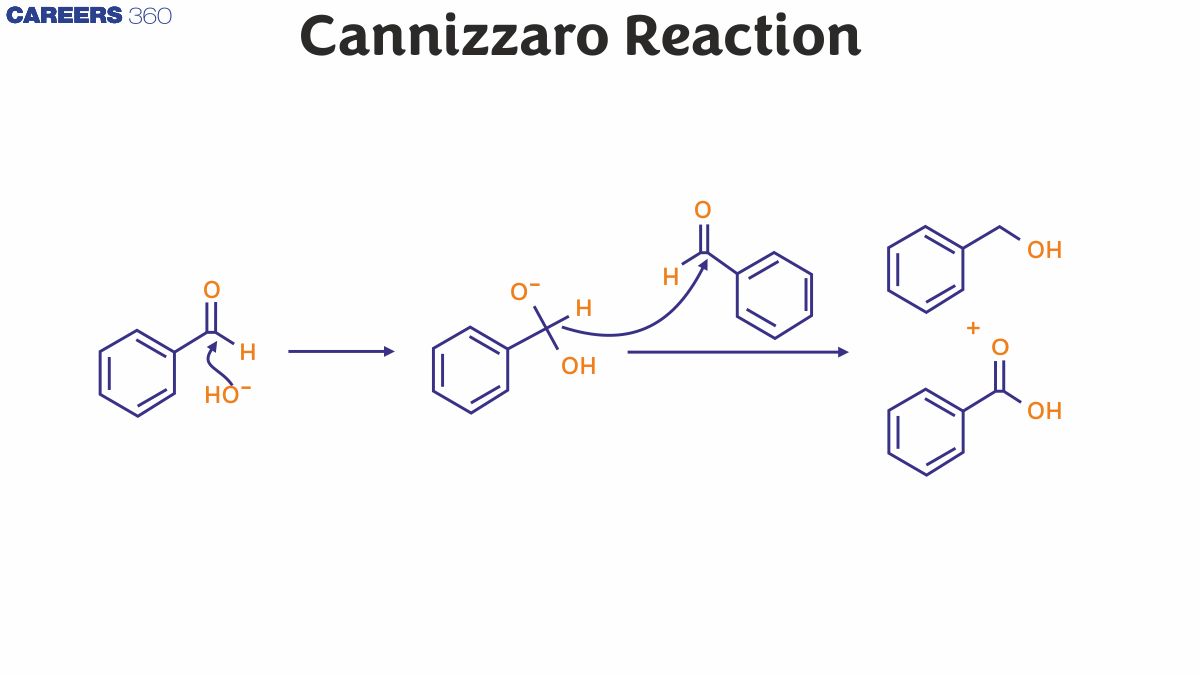
Cannizzaro reaction examples include vanillin, benzaldehyde, syringaldehyde, and formaldehyde, which lack active hydrogen. They are subjected to a strong base (NaOH) intramolecular and intermolecular oxidation process to create a carboxylic acid and alcohol molecule
Cannizzaro Reaction Mechanism
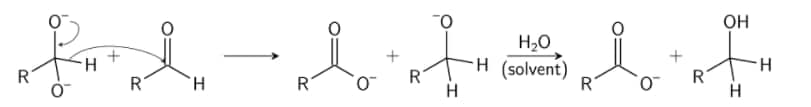
Cannizzaro reaction mechanism describes in detail the way of obtaining one alcohol molecule and one carboxylic acid molecule from two aldehyde molecules. Scientist Stanislao Cannizzaro acquired benzyl alcohol and potassium benzoate from benzaldehyde in 1853. An aldehyde is replaced by a nucleophile in the leaving group, where the other aldehyde is attacked. The consequence of a hydroxide attack on a carbonyl is a tetrahedral intermediate. This intermediate tetrahedral collapses and reforms, allowing the carbonyl to move and a hydride to attack another molecule.
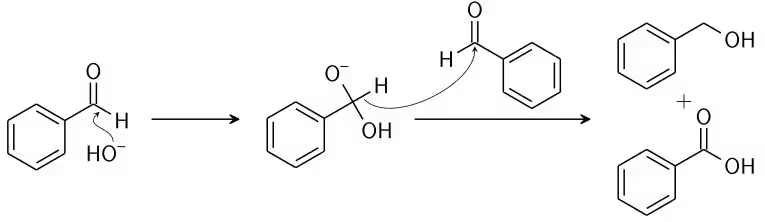
Now, an acid-and-alcoholic ion proton is swapped. The aldehyde generates an anion with a charge of 2 if a base with high concentration is supplied. A hydride ion, which is made of carboxylate and alcohol, is transmitted to a second molecule of the aldehyde. The alcohol ion additionally acquires a proton for the reaction from the solvent.
Mechanism of Cannizzaro Reaction
Step 1: A nucleophile, as hydroxide, is utilised to attack the group of carbonyl in the aldehyde in question, which causes a disproportionate reaction and leads to a two negative charges carrying anion.
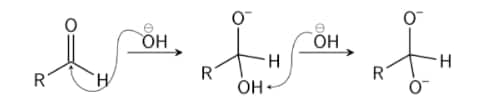
Step 2: This intermediate product can now work as a reduction in hydride. The intermediate releases a hydride anion because of its unstable nature. This anion hydride attacks another molecule of aldehyde. Now, the doubly charged anion becomes an anion for carboxylate and the aldehyde becomes anion for alcohol.
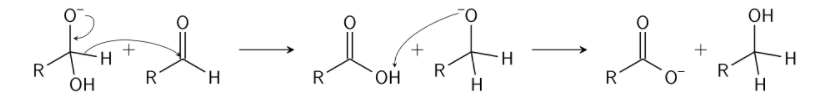
Step 3: In this final stage, water provides the alcohol anion with a proton that creates the final alcohol product. The reaction might take place because the alcohol is basic rather than water. Now, when acid is utilised, the carboxylate ion creates the ultimate product of carboxylic acid (the acid workup is required since carboxylate is less basic than water and therefore cannot obtain a proton from water).
In general, the response follows kinetics in the third order. In aldehyde it is second and in basic it is first:
Temporary =$\mathrm{k}[\mathrm{RCHO}]^2\left[\mathrm{OH}^{-}\right]$
A second way (k') is necessary for very high foundations, which is second order in base:
Temporary rate = k[RCHO]
$2[\mathrm{RCHO}]+\mathrm{k}^{\prime}\left[\mathrm{OH}^{-}\right]^2$
A reaction involving doubly charged anion $\left(\mathrm{RCHO}_2{ }^{2-}\right)$ and aldehyde is involved in the k' trajectory. Direct transmission of hydride ions is obvious from the finding that no deuterium is bound to the α-carbon in the recuperated alcohol when the reaction is carried out when$\mathrm{D}_2 \mathrm{O}$ occurs.
Crossed reaction of Cannizzaro
Crossed Cannizzaro reaction is not unexpected that only 50% of the alcohol and carboxylic acid necessary in the optimal conditions is produced by the reaction. Therefore, the crossover reaction of Cannizzaro is more widely utilised. Sacrificial aldehyde and a more valuable molecule are mixed, and sodium oxidation is reduced by formaldehyde. Reduction of other aldehyde chemicals obtains the necessary alcohol. The yield of the useful chemistry is boosted when two separate aldehydes can be fully transformed into the needed product. Finally, it can be utilised to disproportionate a non-enolizable aldehyde with the Cannizzaro reaction. The reaction from the cross cannizzaro reaction is used to boost the yield of the precious product.
Scope of Cannizzaro reaction:
Aldehydes with alpha hydrogen atom(s) have been decongested, causing enolates and potential aldol reactions, due to very alkaline reactions. The process only yields 50% alcohol and carboxylic acid under optimal conditions (it takes two aldehydes to produce one acid and one alcohol). In order to prevent low yields, the crossing Cannizzaro reaction, which combines sacrificial aldehyde with a more valuable molecule, can be conducted more frequently.
In this variant, formaldehyde is the reducer that is oxidised to formate sodium while alcohol is reduced to the other aldehyde molecule. In this case, one aldehyde can be converted to its matching product fully, instead of losing 50% of a single reactant for each of two products. Therefore, while the atomic economy is still low, the valuable chemical's output is high.
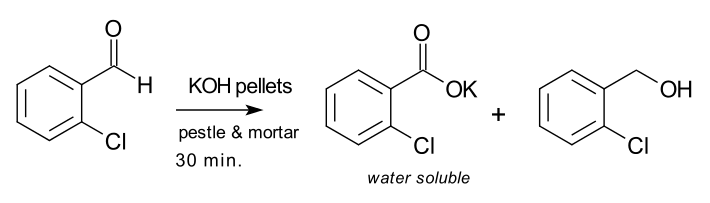
There has been a solvent-free process, including the grinding of fluid 2-chlorobenzaldehyde in a mortar and pestle by potassium hydroxide:
Also read :
Cannizzaro Reaction Application
In the industry, polyols are made via the combination of crossed Cannizzaro reactions and aldol condensation. Polyols are very valuable and they have numerous industrial uses.
-
For the production of resins in the aeroplane or on board, varnish coatings, synthetic lubricants and plasticizers, Neopentyl glycol is employed in polyesters. The structure of neopentyl provides high light, heat and hydrolysis resistance.
-
As a raw ingredient, pentaerythritol is explosive in the varnish industry. Some esters of pentaerythritol are utilised as oil additives, plastifying agents and emulsifiers with higher fatty acids.
-
In a number of applications, Trimethylolpropane is employed for alkaline resin and polyester and polyurethane preparation as replacement glycerine.
Also read -
Some Solved Examples
Question 1: Which one of the following reactions will not result in the formation of a carbon-carbon bond?
1) Reimer-Tieman reaction
2) Friedel-Craft’s acylation
3) Wurtz reaction
4) (correct) Cannizzaro reaction
Solution:
Cannizzaro's reaction -
Shown by aldehydes that do not have $\alpha$ - H atom and undergo a disproportion (self-oxidation reduction) process to produce alcohol and carboxylic acid salt.
$2 \mathrm{HCHO} \xrightarrow{\text { conc. } \mathrm{NaOH}} \mathrm{HCOONa}+\mathrm{CH}_3 \mathrm{OH}$
In the Cannizzaro reaction, two molecules of an aldehyde react to produce a primary alcohol and a carboxylic acid using a hydroxide base.
Hence, the answer is option (4).
Question 2: Which of the following reactions will not result in the formation of carbon-carbon bonds?
1) Reimer-Tieman reaction
2) (correct) Cannizaro reaction
3) Wurtz reaction
4) Friedel-Crafts acylation
Solution:
Hence, the answer is option (2).
Question 3: Tischenko reaction is a modification of :
1) Aldol condensation
2) Claisen condensation
3) (correct) Cannizzaro reaction
4) Pinacol-pinacolone reaction
Solution:
Tishchenko reaction -
In the presence of aluminum ethoxide, aldehydes form esters.

Cannizzaro's reaction -
It is shown by aldehydes that do not have $\alpha-\mathrm{H}$ atom and undergo a disproportion (self-oxidation reduction) process to produce alcohol and carboxylic acid salt.
$2 \mathrm{HCHO} \xrightarrow{\text { conc. } \mathrm{NaOH}} \mathrm{HCOONa}+\mathrm{CH}_3 \mathrm{OH}$
Both are disproportionation reactions (one species undergoes both oxidation and reduction)
Hence, the answer is option (3).
Question 4: Trichloroacetaldehyde was subjected to Cannizzaro’s reaction by using NaOH. The mixture of the products contains sodium trichloroacetate ion and another compound. The other compound is
1) (correct) 2,2,2-trichloroethanol
2) trichloromethanol
3) 2,2,2-trichloropropanol
4) chloroform
Solution:
Cannizzaro's reaction -
Shown by aldehydes that do not have $\alpha-\mathrm{H}$ atom and undergo a disproportion (self-oxidation reduction) process to produce alcohol and carboxylic acid salt.
Cannizzaro product of the given reaction yields 2,2,2-trichloroethanol

Hence, the answer is option (1).
Practice more questions with the link given below
Frequently Asked Questions (FAQs)
Primary alcohols and carboxylic acids generated in a Cannizzaro reaction.
The yield of the intended product is improved by this adjustment of the reaction. Both aldehydes are completely converted to products, avoiding the waste of valuable reactant chemicals. The process's atom economy is likewise low.
The alkaline environment causes the alpha-hydrogen to deprotonate. Due to its three alpha hydrogen, acetaldehyde rapidly generates enolates ions when deprotonate and so cannot participate in the process.
The potassium carboxylate salt or the sodium carboxylate salt of the corresponding carboxylic acid is formed when potassium hydroxide or sodium hydroxide is used in the base-induced disproportionation process.

