Etard Reaction Mechanism - Overview, Application, Limitation, FAQs
Have you ever wondered how chemists selectively convert a simple toluene into a benzaldehyde without oxidizing it to benzoic acid? Why is the presence of chromyl chloride $\left(\mathrm{CrO}_2 \mathrm{Cl}_2\right)$ so simple? We will find all these answers by studying this article on Etard oxidation. Etard oxidation is a transformation where chromyl chloride oxidizes the $-\mathrm{CH}_3$ group of aromatic compounds like toluene into the –CHO group, forming benzaldehyde.
This Story also Contains
- Etard Reaction
- Etard Reaction Mechanism
- Applications of Etard Reaction
- Limitations of Etard Reaction
- Some Solved Examples
Etard Reaction
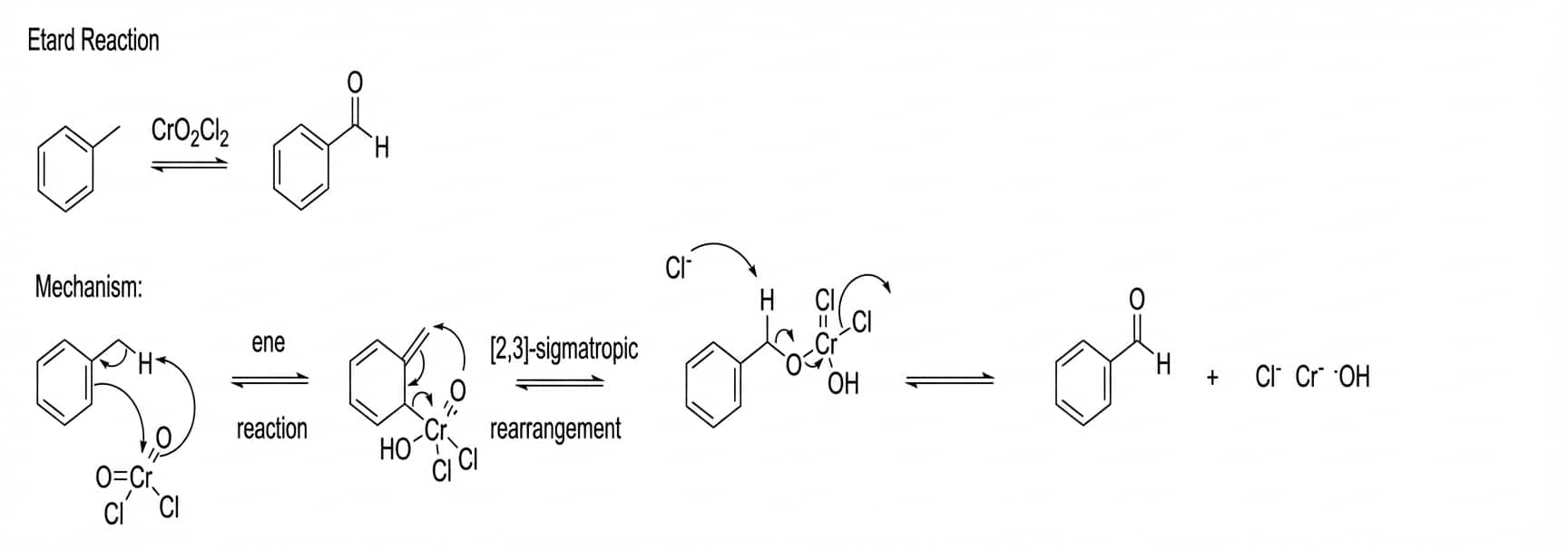
Etard reaction in chemistry is the partial oxidation of a methyl group linked to an aromatic ring with C and a non-polar solvent (such as carbon tetrachloride, carbon disulphide , etc.) to yield an aldehyde. The following reaction is-

The Etard complex is formed when an alkene – allylic hydrogen reaction occurs with chromyl chloride, resulting in an alkene – allylic hydrogen reaction. To prevent the Etard complex from oxidizing into a carboxylic acid, it is destroyed in a reducing environment. A saturated solution of aqueous sodium sulphite is commonly used to create this reducing environment. The most frequent solvent for this procedure is carbon tetrachloride; however, carbon disulfide and chloroform can also be employed. Purifying the Etard complex before breakdown results in a high-purity aldehyde product (so that its reaction with the unreacted reagent is prevented). This equation can take anything from a few days to several weeks to complete, but the yields are relatively high.
Read more :
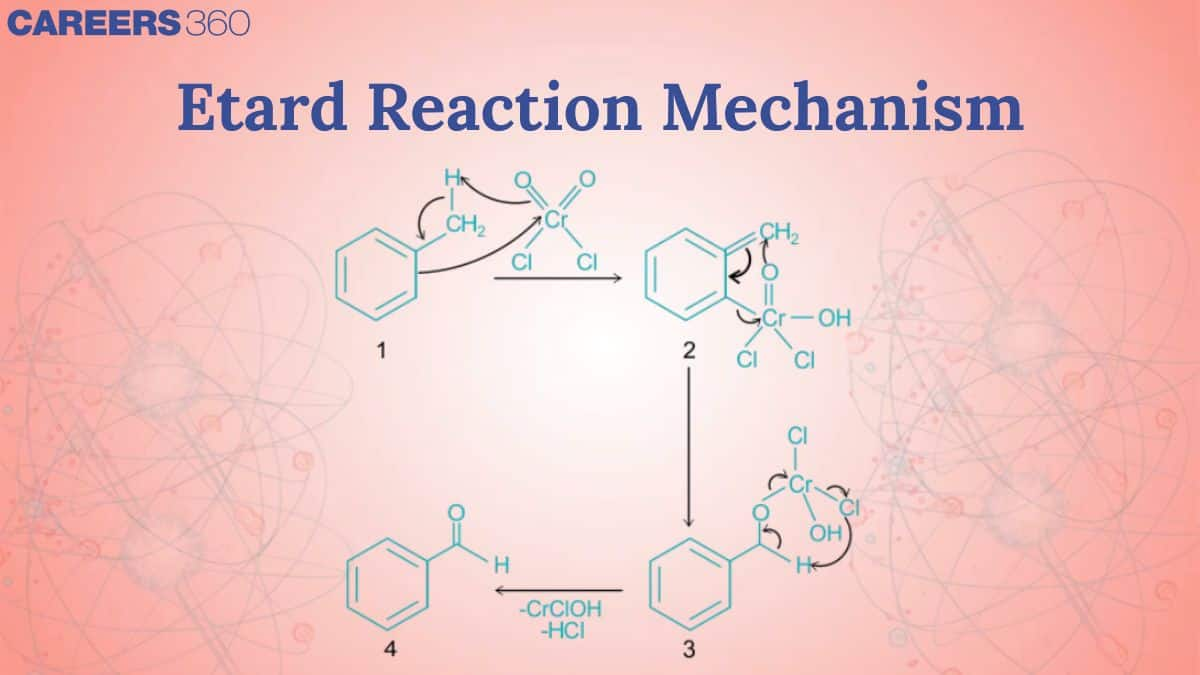
Etard Reaction Mechanism
In the Etard reaction, chromyl chloride, a mild oxidizing agent, interacts with toluene in the presence of carbon tetrachloride, a non-polar solvent. The - bonds of chromyl chloride are cleaved homolytically during this reaction. Similarly, homolytic breakage of the methyl group's C-H bonds occurs. The Etard complex, also known as the chromyl complex, is formed as a result of this reaction. Now the etard complex is hydrolyzed, resulting in the elimination of two molecules of Cr(OH)2Cl2 and the creation of benzaldehyde. Aldehyde is formed by direct partial oxidation of the methyl group linked to the aromatic ring. Etard reaction equation:
|
Related Topics Link, |
Applications of Etard Reaction
The conversion of toluene to benzaldehyde by oxidation is particularly beneficial in the food business because benzaldehyde has an almond-like flavour. It's used to make colours, fragrances, and a variety of pharmaceutical chemicals. Aldehydes are more reactive and participate in the formation of aldols. Many chemicals, including phentermine, can be made from benzaldehyde.
Limitations of Etard Reaction
Although the Etard reaction is a quick and easy way to convert toluene to benzaldehyde. It does, however, have some restrictions. It's difficult to get particular aldehyde products using other reagents than toluene in an etard reaction. When powerful oxidizing agents are used in the process, more stable carboxylic acids are produced.
Also check-
Some Solved Examples
Question 1: Which reagent is used in the Etard Reaction for the selective oxidation of toluene to benzaldehyde?
A) Potassium permanganate
B) Chromyl chloride $\left(\mathrm{CrO}_2 \mathrm{Cl}_2\right)$
C) Dichromate + $\mathrm{H}_2 \mathrm{SO}_4$
D) Ozone
Solution:
The Etard reaction specifically employs chromyl chloride to oxidize the –CH₃ group of aromatic hydrocarbons into –CHO selectively. Strong oxidizers like KMnO₄ would oxidize it further to benzoic acid.
Hence, the correct answer is option is B) Chromyl chloride $\left(\mathrm{CrO}_2 \mathrm{Cl}_2\right)$
Question 2: In the Etard reaction, what is the immediate product formed when toluene reacts with chromyl chloride?
A) Benzaldehyde
B) Chromyl toluate complex
C) Benzoic acid
D) Benzyl alcohol
Solution:
The first step is the formation of a complex between chromyl chloride and the benzylic hydrogen of toluene. On hydrolysis, this complex yields benzaldehyde.
Hence, the correct answer is option is B) Chromyl toluate complex
Question 3: Which of the following aromatic compounds can undergo the Etard Reaction?
A) Toluene
B) Ethylbenzene
C) Chlorobenzene
D) Nitrobenzene
Solution:
Etard reaction requires a $-\mathrm{CH}_3$ group directly attached to the benzene ring. Substituents like nitro or chloro groups without a benzylic carbon cannot undergo this transformation.
Hence, the correct answer is option is A) Toluene
Question 4: The Etard reaction is used to convert:
A) Alkyl benzene into benzoic acid
B) Alkyl benzene into aldehyde
C) Alkyl benzene into ketone
D) Alkyl benzene into alcohol
Solution:
The Etard reaction oxidises the benzylic methyl group $\left(-\mathrm{CH}_3\right)$ of an alkyl benzene to an aldehyde $(-\mathrm{CHO})$ using chromyl chloride $\left(\mathrm{CrO}_2 \mathrm{Cl}_2\right)$.
Hence, the correct answer is option is (B)
Question 5: Which of the following compounds does NOT undergo the Etard reaction?
A. Toluene
B. Ethylbenzene
C. Isopropylbenzene
D. Chlorobenzene
Solution:
The Etard reaction requires a benzylic hydrogen.
Chlorobenzene has no alkyl side chain, so it does not undergo the reaction.
Hence, the correct answer is option is (D)
Practice More Questions With The Link Given Below
| Methods of Preparation of Aldehydes and Ketones Practice Questions and MCQs |
| Reduction and Oxidation Reaction of Aldheyde and Ketones Practice Questions and MCQs |
Frequently Asked Questions (FAQs)
Chromyl Chloride is the reagent employed (CrO2Cl2). It's also known as Etard reagent and is a mild oxidizing agent.
Toluene can be transformed to benzoic acid by fully oxidizing it with a powerful oxidizing agent such as KMnO4.
Toluene can be converted to Benzaldehyde by combining it with Chromyl Chloride in a CS2 and CCl4 medium as well as then hydrolyzing intermediate product.
The partial oxidation of aromatic ring with attached methyl group to create desirable aldehydes is known as the Etard reaction.
In the Etard reaction, chromyl chloride, a mild oxidizing agent, interacts with toluene in the presence of carbon tetrachloride, a non-polar solvent. The - bonds of chromyl chloride are cleaved homolytically during this reaction. Similarly, homolytic breakage of the methyl group's C-H bonds occurs. The Etard complex, also known as the chromyl complex, is formed as a result of this reaction.
Now the etard complex is hydrolyzed, resulting in the elimination of two molecules of Cr(OH)2Cl2 and the creation of benzaldehyde. Aldehyde is formed by direct partial oxidation of the methyl group linked to the aromatic ring.
The conversion of toluene to benzaldehyde by oxidation is particularly beneficial in the food business because benzaldehyde has an almond-like flavour. It's used to make colours, fragrances, and a variety of pharmaceutical chemicals. Aldehydes are more reactive and participate in the formation of aldols. Many chemicals, including phentermine, can be made from benzaldehyde.
