Carbonic Acid - Structure, Importance, Properties & Uses, FAQs
Have you ever wondered how carbon dioxide dissolved in water forms an acid? What is the structure of the weak acid responsible for acidity in rainwater and blood buffering systems? you will find these answers by reading this article on carbonic acis. Carbonic acid has a chemical formula $\mathrm{H}_2 \mathrm{CO}_3$. There is a small amount of this compound in the solution of carbon dioxide in water. Since the compound contains one carbon-oxygen double bond, its chemical formula is (H2CO3). The carbon dioxide chemical formula is CO2. As the only acid exhaled in its gaseous state by the lungs, carbonic acid is often described as a respiratory acid. Carbonate and bicarbonate salts are formed by it; it is a weak acid.
This Story also Contains
- Carbonic Acid Structure
- Carbonic Acid Properties
- 1. Physical-Chemical Properties
- 2. Chemical Properties
- Carbonic Acid Uses
- Importance of Carbonic Acid in Oceans
- Some Solved Examples
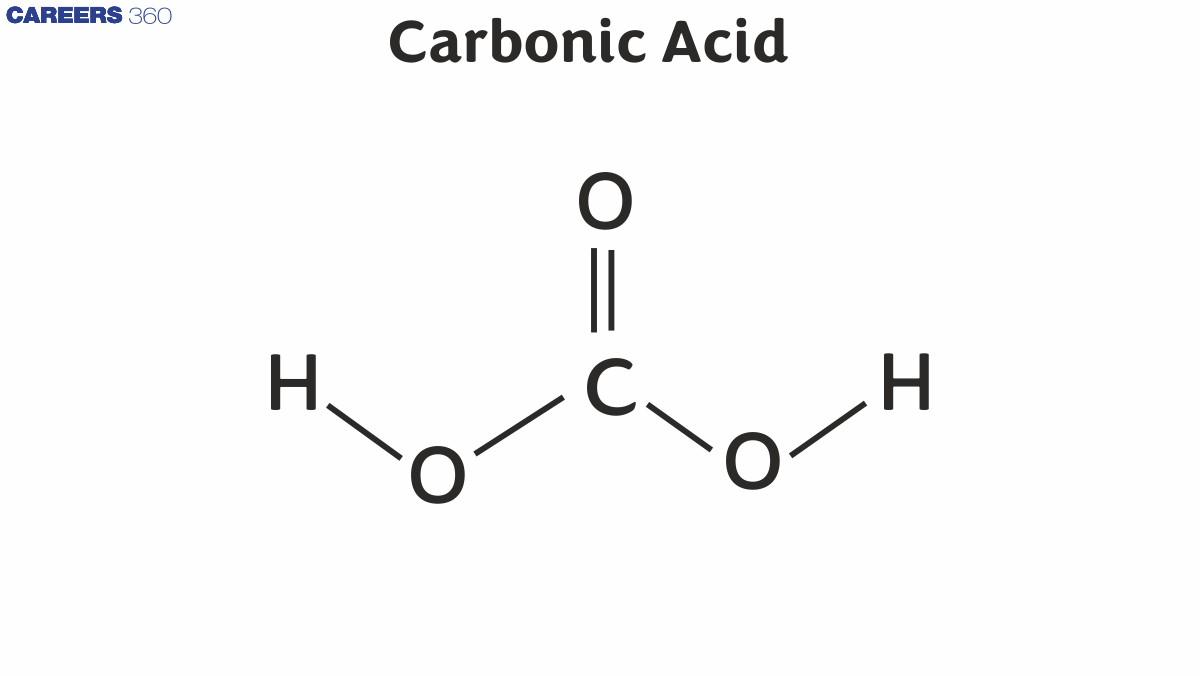
Carbonic Acid Structure
Carbonic acid has a trigonal planar arrangement around the carbon atom.
$\mathrm{HO}-\mathrm{C}(=\mathrm{O})-\mathrm{OH}$
- Central atom: Carbon (C)
- Bonds:
- One double bond between carbon and oxygen ( $\mathrm{C}=\mathrm{O}$ )
- Two single bonds between carbon and hydroxyl groups (-OH)
- Hybridization of carbon: $s p^2$
- Bond angle: $\sim 120^{\circ}$
- Shape: Trigonal planar around carbon
Carbonic acid is unstable in free form and usually exists in equilibrium with carbon dioxide and water:
$\mathrm{H}_2 \mathrm{CO}_3 \rightleftharpoons \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}$
As demonstrated by the illustration above, carbonic acid has a structure that includes a carbon-oxygen double bond and two carbon-oxygen single bonds. One Hydrogen atom is attached to each oxygen atom participating in a single bond with the carbon.
Also read :
Carbonic Acid Properties
Under this subsection, you will find a list of some of the significant physical and chemical properties of carbonic acid.
1. Physical-Chemical Properties
- Carbonic acid has a molar mass of 62.024 grams per mole.
- 1.668 grams per cubic centimeter is its density in its standard state.
- In chemical terms, $\mathrm{H}_2 \mathrm{CO}_3$ has a p Ka of 6.35.
- As a conjugate base, bicarbonate corresponds to carbonic acid.
- In most cases, this compound is dissolved in water. There have been reports that NASA scientists have prepared solid $\mathrm{H}_2 \mathrm{CO}_3$ samples.
2. Chemical Properties
- A weak acid, $\mathrm{H}_2 \mathrm{CO}_3$ is inherently unstable in nature.
- When water is present, it dissociates partially to give H+ and $\mathrm{HCO}_3^{-}$ (bicarbonate) ions.
- A diprotic acid such as carbonic acid can form two types of salts, both of which are bicarbonates.
- Bicarbonate salts are formed by adding a small quantity of a base to $\mathrm{H}_2 \mathrm{CO}_3$, whereas carbonate salts are formed by adding an excess of a base.
- Interestingly, industrial fermentation processes or the burning of fossil fuels can result in the production of carbonic acid by-products.
Carbonic Acid Uses
With a wide range of uses, $\mathrm{H}_2 \mathrm{CO}_3$ is a very useful compound. Carbonic acid is also used in the following ways.
- It is carbonic acid that is used to prepare carbonated water, sparkling wine, and other aerated drinks.
- Ammonium persulfate is precipitated from $\mathrm{H}_2 \mathrm{CO}_3$ powder.
- By transporting carbon dioxide from the body, it helps to eliminate it.
- Ringworm and other dermatitis are treated by protonating certain nitrogenous bases in blood serum and applying a solution of carbonic acid to the area.
- Cleansing contact lenses with these solutions is extremely effective.
- When necessary (such as during drug overdoses), it can be consumed orally to induce vomiting.
Importance of Carbonic Acid in Oceans
oceans are believed to have shifted the pH of the ocean water by approximately 0.1 due to the absorption of excess carbon dioxide from the atmosphere (primarily caused by human activities). Ocean water reacts with carbon dioxide to form hydrogen carbonate. Ocean acidification is a common term used to describe this process.
Also check-
Some Solved Examples
Question 1: Carbonic acid exists in aqueous solution mainly due to:
A. Strong hydrogen bonding
B. High stability of $\mathrm{H}_2 \mathrm{CO}_3$
C. Equilibrium between $\mathrm{CO}_2$ and $\mathrm{H}_2 \mathrm{O}$
D. Complete ionization in water
Solution:
Carbonic acid is unstable and exists only in equilibrium:
$\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}_2 \mathrm{CO}_3$
Hence, it survives only due to continuous formation from $\mathrm{CO}_2$ and water.
Hence, the correct answer is option (3)
Question 2: The hybridization and geometry of carbon atom in carbonic acid are:
A. $\mathrm{sp}^3$, tetrahedral
B. $\mathrm{sp}^2$, trigonal planar
C. sp, linear
D. $\mathrm{sp}^3$, pyramidal
Solution:
Carbon forms:
- One $\mathrm{C}=\mathrm{O}$ double bond
- Two C-O single bonds
Total $3 \sigma$ bonds $\rightarrow \mathrm{sp}^2$ hybridization, giving trigonal planar geometry.
Hence, the correct answer is option (2)
Question 3: Which of the following correctly represents the structure of carbonic acid?
A. $\mathrm{O}=\mathrm{C}(\mathrm{OH})_2$
B. $\mathrm{HO}-\mathrm{C} \equiv \mathrm{O}-\mathrm{OH}$
C. $\mathrm{HO}-\mathrm{C}(-\mathrm{OH})=\mathrm{O}$
D. $O-C(=O)-O$
Solution:
Carbonic acid structure is:
$\mathrm{HO}-\mathrm{C}(=\mathrm{O})-\mathrm{OH}$
Hence, the correct answer is option (1)
Question 4: Carbonic acid behaves as:
A. Monobasic acid
B. Dibasic acid
C. Tribasic acid
D. Neutral compound
Solution:
Carbonic acid has two replaceable hydrogen atoms, hence it is a dibasic (diprotic) acid:
$\mathrm{H}_2 \mathrm{CO}_3 \rightarrow \mathrm{HCO}_3^{-} \rightarrow \mathrm{CO}_3^{2-}$
Hence, the correct answer is option (2)
Frequently Asked Questions (FAQs)
Besides soft drinks, artificially carbonated sparkling wines, and other bubbly beverages, carbonic acid is also used to make artificially carbonated sparkling wine. Bicarbonates (or hydrogen carbonates) and carbonates are the salts of carbonic acid.
Carbonic acid is a hydroxide that contains substituted hydroxyl groups. Additionally, it is a polyprotic acid. Since this compound has two protons, it is diprotic and therefore belongs to a group of chemicals called diprotic compounds. The dynamics of dissociation can be described using two constants, where the first is for dissociation into the bicarbonate ion.
Carbon dioxide is expelled from the body by bicarbonate, a chemical intermediate. Generally, CO2 does not hydrate fast without a catalyst, but red blood cells release a substance known as carbonic anhydrase, which speeds up the metabolism of CO2, forming dissolved bicarbonate (HCO3– ) in the blood plasma.
A strong acid is a carbonic acid, not carbonic acid itself. Weak acids such as H2CO3 are dissociated into proton-containing cations (H+ cations) and bicarbonate-containing anion-forming cations (HCO3– cations). The compound does not completely dissociate in water. As a matter of fact, the bicarbonate ion of carbonic acid, which is the conjugate base of carbonic acid, is a relatively good base. Due to these reasons, carbonic acid is classified as a weak acid rather than as a strong acid.
Carbonic acid is formed when carbon dioxide gas dissolves in water. The reaction can be represented as:
[$ \text{CO}_2 + \text{H}_2\text{O} \rightleftharpoons \text{H}_2\text{CO}_3 $]
This equilibrium can shift based on factors like pressure and temperature.
