Benzoic Acid - Overview, Structure, Preparation, Properties & Uses, FAQs
Benzoic acid is an organic compound with $\mathrm{C}_6 \mathrm{H}_5 \mathrm{COOH}$ as its chemical formula. It consists of a benzene ring and a carboxylic group. The compound exists in normal conditions as a colourless crystalline solid. Benzoic acid is an organic compound that is used in our daily lives for various food and cosmetic products. It is used in many products such as pickles, jams, lipsticks, and wash creams, among others. In addition, it is an essential precursor in many organic combination syntheses.
This Story also Contains
- Benzoic Acid Structure
- Preparation of Benzoic Acid
- Resonating Structure of Benzoic Acid
- Physical Properties of Benzoic Acid
- Chemical Properties of Benzoic Acid
- Uses of Benzoic Acid
- Some Solved Examples
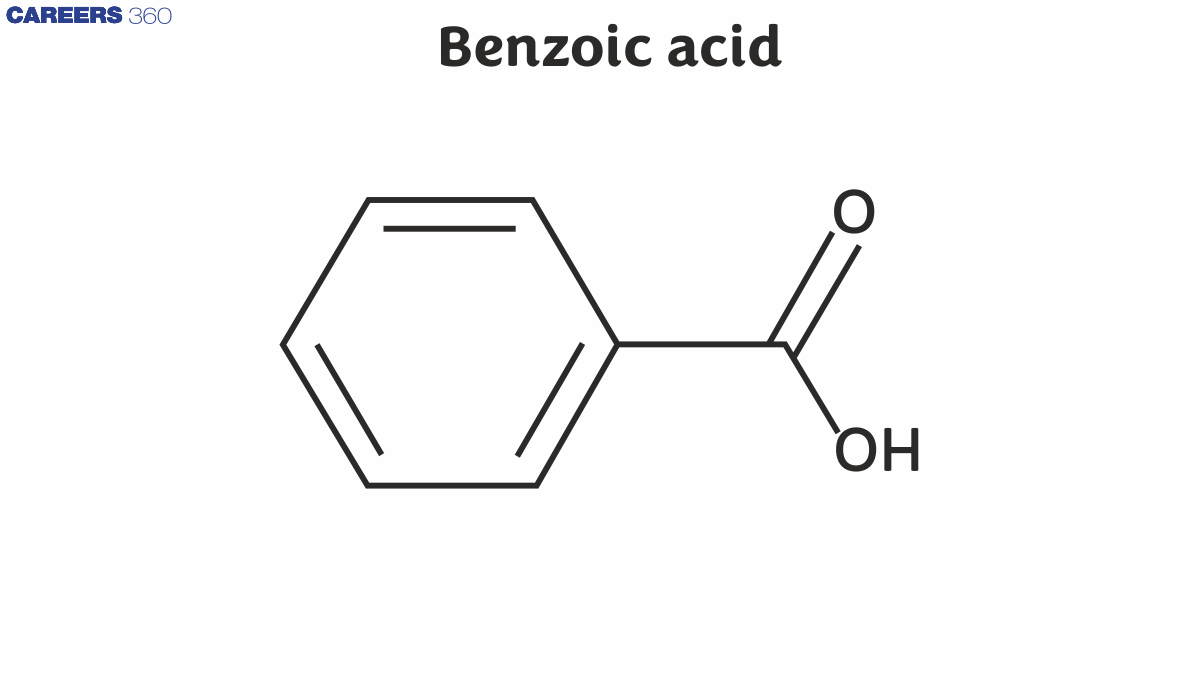
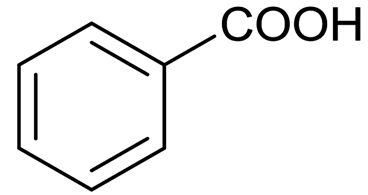
Benzoic Acid Structure
Benzoic acid is also known as Benzene carboxylic acid. There are 7 carbon atoms, 2 oxygen atoms, and 6 hydrogen atoms in its structure. It consists of a six-carbon ring with alternate single and double bonds, as well as the group of –COOH attached to the ring that makes it benzoic acid.
Benzoic acid formula: C6H5COOH
The benzoic acid structure is given below:
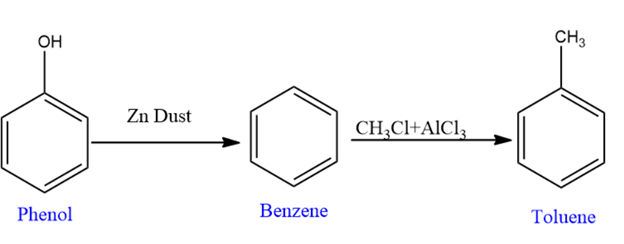
Preparation of Benzoic Acid
In the process of preparing benzoic acid, we convert Phenol to benzoic acid.
First, we convert phenol to benzene using zinc dust, and then we convert benzene to toluene using the $\mathrm{CH}_3 \mathrm{Cl}+\mathrm{AlCl}_3$, where aluminum trichloride acts as Lewis acid catalyst.
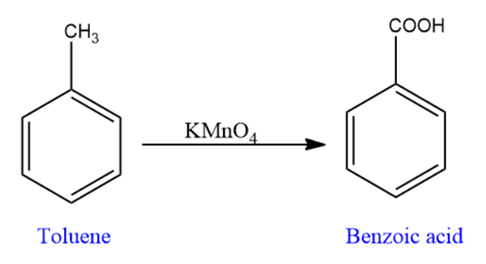
Then, Toluene is converted into benzoic acid by oxidation.
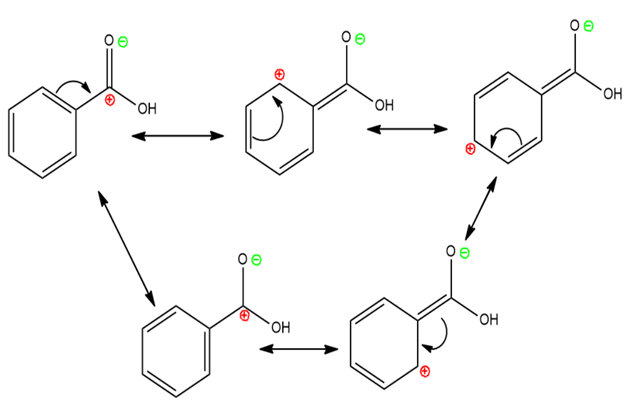
Resonating Structure of Benzoic Acid
$\mathrm{COOH}$ shows $-\mathrm{M}(-\mathrm{R})$ effect $\rightarrow$ Withdraws electrons from the ring via resonance.
Decreases electron density at ortho and para positions $\rightarrow$ Makes benzoic acid meta-directing for electrophilic substitution.
Resonance stabilizes the acid form $\rightarrow$ Stronger acid than alcohols.
|
Related Topics |
Physical Properties of Benzoic Acid
- In its solid state of crystalline nature, benzoic acid has an uncoloured appearance. It is nonclinical in its crystal structure.
- This compound is slightly pleasant because of the aromatic ring.
- This compound's density decreases to 1,075 grams per cubic centimeter at a temperature of $130^{\circ} \mathrm{C}$.
- The melting point of benzoic acid is $122.3^{\circ} \mathrm{C}$.
- Benzoic acid molar mass - 122.12 g/mol.
Chemical Properties of Benzoic Acid
- The solubility is 3.44 g/L and 56.31 g/l, respectively, at a temperature range of $25^{\circ} \mathrm{C}$ and $100^{\circ} \mathrm{C}$.
-
Benzene, carbon tetrachloride, acetone, and alcohol are soluble.
-
The benzoic acid pka dissociation constant is 4.2.
-
The carboxyl group or even the aromatic Ring may experience its reactions.
Uses of Benzoic Acid
It is used in an acidic medium, but it has lost preservation activity in an alkaline medium because it divides into ions in an alkaline medium.
-
The use of benzoic acid as a preservative does not affect the odour or taste of food products.
-
It delays the reproduction of microorganisms by preserving the quality.
-
The benzoic acid sodium salt, sodium benzoate, reduces the blood level of glycine because it forms an amide bond between the benzoate and glycine that is excreted by the urinary tract.
-
Some benzoic acid esters act as plasticizers.
-
For many foodstuffs, such as soda, barbecue sauces, pickles, and dressings, which prevent the growth of bacteria and fungi, benzoic acid and its sodium salt are used as food preservatives.
-
Benzoic acid is used to treat skin irritation, sunburns, insect bites, and fungal infections. This is used specifically in cosmetics.
-
It is used as a pH adjuster in the preparation of fragrances.
-
It is mostly used in acidic foods because even at very low pH, it has more anti-food and antibacterial properties.
Also Read
| NCERT Solutions for Class 11 Chemistry | ||
| NCERT Solutions for Class 12 Chemistry | ||
| NCERT Solutions for All Subjects |
Some Solved Examples
Question 1: Which of the following does not form Benzoic acid upon hydrolysis?
1) Benzonitrite
2) (correct) Benzyl chloride
3) Benzoyl chloride
4) Ethylbenzoate
Solution:
The hydrolysis of the given compound occurs

Hence, the answer is option (2).
Question 2: The compound formed as a result of the oxidation of ethyl benzene by is
1) benzyl alcohol
2) benzophenone
3) acetophenone
4) (correct) benzoic acid.
Solution:
oxidation of Alkylbenzene -
The entire side chain of alkylbenzene is oxidised to the carboxyl group, irrespective of the length of the side chain.
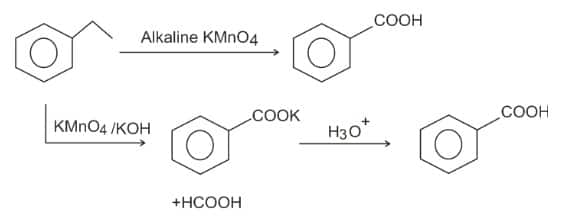
The product is benzoic acid.
Hence, the answer is option (4).
Question 3: An aromatic compound 'A' having molecular formula $\mathrm{C}_7 \mathrm{H}_6 \mathrm{O}_2$ on treating with aqueous ammonia and heating forms compound ' B '. The compound ' B ' on reaction with molecular bromine and potassium hydroxide, provides compound 'c' having molecular formula $\mathrm{C}_6 H_7 N$. The structure of 'A; is:
1)
2)
3)
4)
Solution:
Esterification of carboxylic acid -
⇒ Carboxylic acid with alcohols or phenols forms esters in the presence of a catalyst H2SO4 / HCl.
⇒ It's a kind of nucleophilic acyl substitution.
⇒ Involves cleavage of the C-OH bond.
$\mathrm{RCOOH}+\mathrm{R}^{\prime} \mathrm{OH} \rightleftharpoons \mathrm{RCOOR}+\mathrm{H}_2 \mathrm{O}$
As we have learned, when aq NH3 acts as a reagent.
A has a molecular formula C7H6O2, so the answer will be -
(Benzoic acid is 'A' )
Hence, the answer is option (4).
Practice more questions with the link given below:
Frequently Asked Questions (FAQs)
Benzoic Acid is a white, crystalline powder having a pleasant odour. It is used to make chemicals which are further used in perfumes and flavourings. It is also used as a food preservative and anti- fungal agent.
It is an aromatic acid, moderately strong white crystalline powder, highly soluble in alcohol, ether, and benzene, but poorly soluble in water.
Another name for benzoic acid is benzenecarboxylic acid.
First we convert benzoic acid to benzoyl chloride using SOCl2 . Then we convert the obtained Benzoyal chloride to benzamide by treating it with NH3 .
Benzoic acid is an organic compound with a chemical formula of C7H6O2 or C6H5COOH.




