Decomposition Reaction - Definition, Examples, Types, Uses, FAQs
Why does calcium carbonate decompose into calcium oxide and carbon dioxide on heating? Can a reaction occur in which energy must be supplied in the form of heat, light, or electricity? You will find these answer by studying decomposition reaction. A decomposition reaction is a type of chemical reaction in which a single compound breaks down into two or more simpler substances, such as elements or smaller compounds. This process often requires an external source of energy, such as heat, light, or electricity, to break the chemical bonds.
This Story also Contains
- Examples of Decomposition Reactions
- Uses of Decomposition Reaction
- Double decomposition reaction
- Types of Decomposition reaction
- Thermolysis
- Ease of Decomposition reaction:
- Some Solved Examples
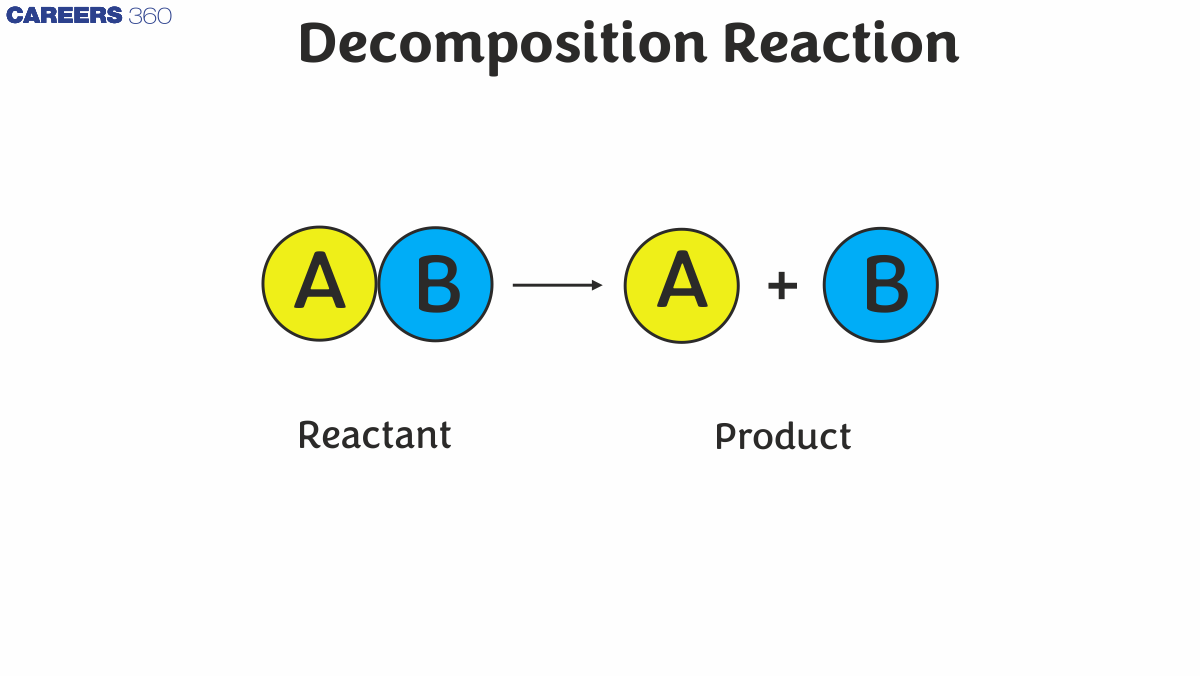
The general formula for a decomposition reaction is: $A B \rightarrow A+B$.
Here, AB is the reactant that decomposes into the simpler products A and B.
Examples of Decomposition Reactions
- Thermal Decomposition:
- Decomposition of calcium carbonate: $\mathrm{CaCO}_3 \rightarrow \mathrm{CaO}+\mathrm{CO}_2$
- Calcium carbonate breaks down into calcium oxide and carbon dioxide when heated.
- Electrolytic Decomposition:
- Electrolysis of water: $2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{H}_2+\mathrm{O}_2$
- Water decomposes into hydrogen and oxygen gas under an electric current.
- Photolytic Decomposition:
- Decomposition of silver chloride: $2 \mathrm{AgCl} \rightarrow 2 \mathrm{Ag}+\mathrm{Cl}_2$
- Silver chloride decomposes into silver and chlorine gas in sunlight.
- Decomposition of hydrogen peroxide: $2 \mathrm{H}_2 \mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_2$
- Hydrogen peroxide decomposes into water and oxygen.
Uses of Decomposition Reaction
The main uses for the decomposition reaction is the extraction of metals from their ores. For example, zinc can be obtained from calamine by placing it in the reaction of decomposition. In the same way, sodium can be found in sodium chloride.
1. Extraction of Metals
Decomposition reactions are used to extract metals from their compounds.
Example:
$\mathrm{CaCO}_3 \xrightarrow{\Delta} \mathrm{CaO}+\mathrm{CO}_2$
2. Manufacture of Quicklime (CaO)
Thermal decomposition of limestone is used in cement and construction industries.
3. Production of Oxygen Gas
Oxygen is prepared in laboratories by decomposition of potassium chlorate or hydrogen peroxide.
$2 \mathrm{KClO}_3 \xrightarrow{\mathrm{MnO}_2} 2 \mathrm{KCl}+3 \mathrm{O}_2$
4. Photographic Industry
Silver bromide and silver chloride decompose in presence of sunlight, forming the basis of black-and-white photography.
$2 \mathrm{AgBr} \xrightarrow{\text { light }} 2 \mathrm{Ag}+\mathrm{Br}_2$
5. Electrolysis of Compounds
Electrolytic decomposition is used to obtain gases and metals like hydrogen, oxygen, and aluminium.
6. Thermal Cracking in Petroleum Industry
Large hydrocarbon molecules decompose into smaller, more useful molecules like petrol and LPG.
7. Chemical Analysis and Testing
Decomposition reactions help in identifying compounds based on the products formed on heating.
8. Recycling and Waste Treatment
Decomposition reactions are used to break down harmful chemical compounds into safer substances.
Double decomposition reaction
A decomposition reaction in which two reactants alternate negative and positive ions and a new chemical is formed is known as double decomposition reaction.
Types of Decomposition reaction
-
Thermal decomposition reaction
The thermal decomposition reaction can be defined as a reaction to decomposition of thermal decomposition. Such reactions are often exhausting because energy is needed to break down chemical bonds and to disassociate.
Example of thermal decomposition reaction of a hot rot reaction is given below.
$\mathrm{CaCO}_3 \rightarrow \mathrm{CaO}+\mathrm{CO}_2$
This process is used in the production of fast lime, which is important in many industries.
-
Electrolyte Decomposition reaction
Electrolytic decomposition chemistry response is a type of decomposition reaction wherein activation power is supplied within the form of electrical electricity. An electrolytic decomposition reaction which is liquid electrolysis
Example of example of electrolytic decomposition reaction:
$2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{H}_2+\mathrm{O}_2$
-
Photochemical decomposition
A photo decomposition reaction is type of decomposition reaction by which reactant is released to builders along with absorbing energy from photons. An example of an image decay response is decay of dioxygen and oxygen radical, as represented by the chemical equation given below.
$\mathrm{O}_3+\mathrm{hv} \rightarrow \mathrm{O}_2+\mathrm{O}$.
|
Related Topics |
Thermolysis
Heat decomposition, or thermolysis, is a chemical decay caused by heat. The decay temperature of an object is the temperature at which the substance decomposes chemically. The reaction often ends as heat is needed to break down the chemical bonds in the decaying area. When decay is disturbing enough, a good reaction loop is created producing a hot escape along with possibly an explosion. Some of the oxides, especially of the less fragile metals, decompose meaning when heated to a sufficient temperature. An ancient example is the decay of mercuric oxide to provide oxygen and mercury metal. When water is heated above 2000°C, a small percentage of it decomposes into OH, monatomic oxygen, monatomic hydrogen, O2, and H2.
The most common decomposing element is carbon monoxide at -3870°C (-7000°F). Decomposition of nitrates, nitrites, and ammonium compounds. Ammonium dichromate in heat produces nitrogen, water, and chromium oxide. Ammonium nitrate at high temperatures produces dinitrogen oxide ("laughing gas") and water. Ammonium nitrite in heat produces nitrogen gas and water. Barium azide in heat produces barium iron and nitrogen gas. Sodium azide at a temperature of 300 ° C provides nitrogen and sodium. Sodium nitrate in heat produces sodium nitrite and oxygen gas. Organic chemicals such as high-temperature amines cause Hofmann to dissolve and produce secondary amines and alkenes.H2O2
Also read :
Ease of Decomposition reaction:
When metals are at the bottom of a series of reactivity, their compounds usually decompose easily at high temperatures. This is because the form of solid bonds between atoms at the top of a series of reactivity, and strong bonds is difficult to break.
For example, copper is near the bottom of a series of reactivity, and copper sulphate begins to decompose at about 200 ° C, rising rapidly at high temperatures to about 560 ° C. In contrast, potassium is close to the top of the series of reactivity, and potassium sulphate does not decompose where it melts at about 1069 ° C, or where it boils.
Also check-
Some Solved Examples
Question 1: Which of the following is Decomposition Redox Reaction.
1) $2 \mathrm{KClO}_3 \rightarrow 2 \mathrm{KCl}+3 \mathrm{O}_2$
2) $2 \mathrm{Mg}+\mathrm{O}_2 \rightarrow 2 \mathrm{MgO}$
3) (correct) $\mathrm{H}_2 \mathrm{O}_2 \rightarrow \mathrm{H}_2 \mathrm{O}+\frac{1}{2} \mathrm{O}_2$
4) $\mathrm{Mg}+2 \mathrm{HCl} \rightarrow \mathrm{MgCl}_2+\mathrm{H}_2$
Solution:
As we learned
Decomposition Reaction -
This is the opposite of combination reaction precisely, a decomposition reaction lead to the breakdown of a compound into two or more components at least one of which must be in an elemental state.
- wherein
$2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \xrightarrow{\Delta} 2 \mathrm{H}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g})$
+1 -2 0 0
Hence, the answer is the option (3).
Question 2: The thermal decomposition of which of the following compounds does not involve a redox change of the metal?
1) (correct) $\mathrm{Pb}\left(\mathrm{NO}_3\right)_2$
2) $\mathrm{FeSO}_4$
3) (correct) $\mathrm{ZnSO}_4$
4) $\mathrm{SnSO}_4$
Solution:
$\begin{aligned} & 2 \mathrm{~Pb}\left(\mathrm{NO}_3\right)_2 \xrightarrow{\Delta} \mathrm{PbO}+2 \mathrm{NO}_2+\frac{1}{2} \mathrm{O}_2 \\ & 2 \mathrm{FeSO}_4 \xrightarrow{\Delta} \mathrm{Fe}_2 \mathrm{O}_3+\mathrm{SO}_2+\mathrm{SO}_3 \\ & \mathrm{ZnSO}_4 \xrightarrow{\Delta} \mathrm{ZnO}+\mathrm{SO}_2+\frac{1}{2} \mathrm{O}_2 \\ & \mathrm{SnSO}_4 \xrightarrow{\Delta} \mathrm{SnO}_2+\mathrm{SO}_2\end{aligned}$
Hence, the answer is the option (1,3).
Question 3: Match the List-I with List-II
|
List-I (Redox Reaction) |
List-II (Type of redox reaction) | ||
| A | $\begin{aligned} & \mathrm{CH}_{4(\mathrm{~g})}+2 \mathrm{O}_{2(\mathrm{~g})} & \xrightarrow[2 \mathrm{H}_2 \mathrm{O}_{(\mathrm{l})}]{\Delta} \mathrm{CO}_{2(\mathrm{~g})}+\end{aligned}$ | I |
Disproportionation reaction
|
| B |
$2 \mathrm{NaH}_{(\mathrm{s})} \xrightarrow{\Delta}$ $2\mathrm{Na}_{(\mathrm{s})}+\mathrm{H}_{2(\mathrm{~g})}$ | II | Combustion reaction |
| C | $\begin{aligned} & \mathrm{V}_2 \mathrm{O}_{5(\mathrm{~s})}+5 \mathrm{Ca}_{(\mathrm{s})} \\ & \xrightarrow{\Delta} 2 \mathrm{~V}_{(\mathrm{s})}+ \\ & 5 \mathrm{CaO}_{(\mathrm{s})}\end{aligned}$ | III | Decomposition reaction |
| D | $\begin{aligned} & 2 \mathrm{H}_2 \mathrm{O}_{2(\mathrm{aq})} \\ & \xrightarrow{\Delta} 2 \mathrm{H}_2 \mathrm{O}_{(\mathrm{l})}+ \\ & \mathrm{O}_{2(\mathrm{~g})}\end{aligned}$ | IV | Displacement reaction |
Choose the correct answer from the options given below :
1) A-II, B-III, C-IV, D-I
2) A-II, B-III, C-I, D-IV
3) A-III, B-IV, C-I, D-II
4) A-IV, B-I, C-II, D-III
Solution:
(A) Combustion of hydrocarbon
(B) Decomposition into gaseous product.
(C) Displacement of ' $V$ ' by ' Ca ' atom.
(D) Disproportionation of $\mathrm{H}_2 \mathrm{O}_2^{-1}$ into $\mathrm{O}^{-2}$ and $\mathrm{O}^{\circ}$ oxidation states.
Hence, the correct answer is option (1).
Question 3: Which of the following statement(s) is/are not true about the following decomposition reaction.
$2 \mathrm{KClO}_3 \rightarrow 2 \mathrm{KCl}+3 \mathrm{O}_2$
(i) Potassium is undergoing oxidation
(ii) Chlorine is undergoing oxidation
(iii) Oxygen is reduced
(iv) None of the species are undergoing oxidation or reduction
1) (correct) (i) and (iv)
2) (iii) and (iv)
3) (ii) and (iii)
4) (i) and (iii)
Solution:
The answer is the option (i) and (iv)
Explanation: The statements above are not true because it is evident in the reaction that, potassium remains in the same oxidation state, and oxygen is being oxidised.
Hence, the answer is the option (1).
Question 4: What would you classify the following reaction as?
$\mathrm{HgO} \rightarrow \mathrm{Hg}+\frac{1}{2} \mathrm{O}_2$
1) Disproportionation Reaction
2) (correct) Decomposition Reaction
3) Metal Displacement Reaction
4) Non-Metal Displacement Reaction
Solution:
As we learned,
Decomposition Reaction -
This is the opposite of a combination reaction. Specifically, a decomposition reaction leads to the breakdown of a compound into two or more components, at least one of which must be in an elemental state.
- wherein
$2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \xrightarrow{\Delta} 2 \mathrm{H}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g})$
+1 -2 0 0
$\mathrm{HgO} \rightarrow \mathrm{Hg}+\frac{1}{2} \mathrm{O}_2$
It is a decomposition reaction since a compound breaks down into two or more components, at least one of which is in an elemental state.
Hence, the answer is the option (2).
Practice More Question with thew link given below
Frequently Asked Questions (FAQs)
A decomposition reaction is a chemical reaction where a single compound breaks down into two or more simpler substances.
Answer: (b)
Decomposition of silver chloride occurs by Sunlight.
The main types of decomposition reactions are
1. Thermal decomposition (triggered by heat),
2. Electrolytic decomposition (triggered by electricity)
3. Photolytic decomposition (triggered by light).
The decomposition of hydrogen peroxide (H2O2) into water and oxygen is a common example, used in household antiseptics.
