Differential Extraction Chromatography - Principle, Types, Applications FAQs
Differential Extraction
Differential extraction is define as the method which is used for the separation of any organic compound which is present in an aqueous solution. For proceeding this process we need that organic solvent whose solubility is more than the water for the compound which we need to separate out. By choosing organic solvent one thing to keep in mind is it should be immiscible with the given aqueous solution so that it is able to make separate layers which can be easily separate out with the help of separating funnel.
By this we can easily separate out the layer of organic compound with the help of distillation or evaporation. Continuous extraction is also possible but only in that case where solubility of compound is less as compare to chosen organic solvent.
Also check-
What is Chromatography?
Chromatography word generally made up by the combination of two Greek words called chroma and graphein where Chroma represents “color” and graphein has the meaning “to write” and hence Chromatography meaning is a color to write. Chromatography definition can be explained as the technique which is used for the separation, purification and also for testing of compounds.
For chromatography the mixture which can be solid or liquid and has to be separated out is kept as a stationary phase and any pure solvent like water or gas of any compound now allows to move slowly over this stationary phase which carry out the components one by one on the basis of their solubility level in that solvent.
Principle of Chromatography
Chromatography is based on the principle of separation method in which an analyte is get combined within a liquid or gaseous phase and this phase is in mobile condition i.e. free to move and further pumped through a stationary phase. Out of these two phases one is hydrophilic i.e. water loving or have tendency to dissolve in water and other is liophilic i.e. fat loving or we can say those which have ability to dissolve in fats, oils or non-polar solvents.
Interaction of the components of the analyte is different for both the phases it generally depends upon the polarity that which one spend more time or which one spend less time on interaction with stationary phase and after that retardation is also seen that which is retarded to greatest or lesser extent. By this principle the two components gets separated.
We can also notice their retention time where retention time is specific time taken by each component which get elutes from the stationary phase. When the components gets passed through the detector i.e. which detect their signals then their signals will be recorded and plotted in the form of chromatograph.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 5 Surface Chemistry
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 5 Surface Chemistry
- NCERT notes Class 12 Chemistry Chapter 5 Surface Chemistry
Types of Chromatography
There are four mains types of chromatography techniques which are discussed as follows:
1. Adsorption chromatography: As the name suggests adsorption chromatography in this adsorption process takes place and during this process different types of components get adsorbed on the adsorbent on the basis of their adsorptivity value. In this process mobile phase is travelled over the stationary phase which moves the components of higher absorptivity to lower distance as compare to lower absorptivity compounds. Adsorption chromatography can be shown as:
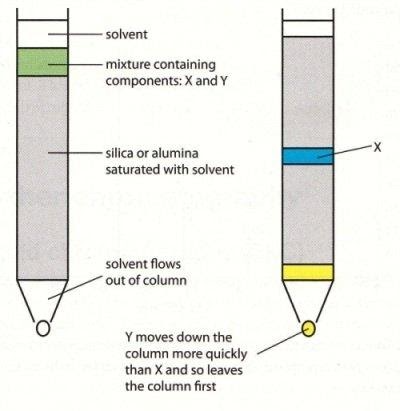
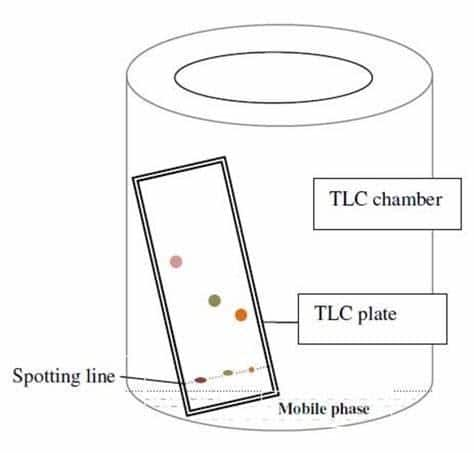
2. Thin layer chromatography: This is one of the most basic technique used in laboratory generally known by its abbreviation T.L.C. In this process mixture of two or more substances get separated into its components by using a glass plate which is coated with silica gel or alumina and this thin layer is act as an adsorbent in this process. Coated glass plate which is used in this process is called chrome plate.
In this process we have to mark a small spot of the mixture of solution which has to be separated at about 2 cm above the end of chrome plate after that put this plate in a close jar which contain that fluid which acts as an eluent which helps to rise the different components of mixture to different heights. Thin layer chromatography technique can be shown as follows:
Related Topics Link, |
3. Column chromatography: It is defined as the technique which is used to separate the components of any mixture with the help of columns containing adsorbent according to their component and these are packed in glass tube and can be shown as follows:
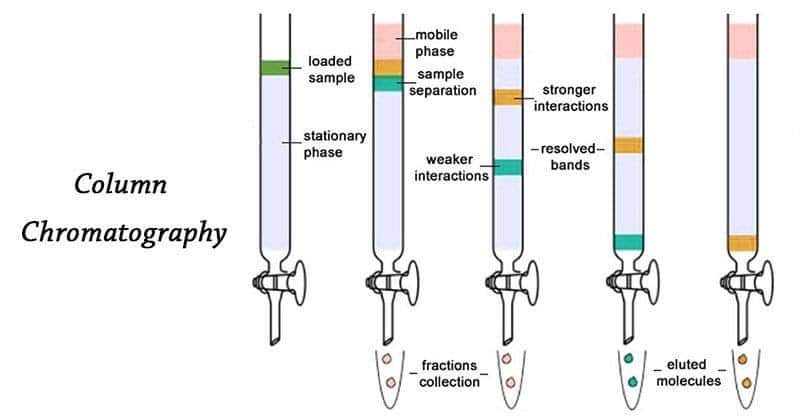
In these columns mixture is placed at top of the tube and eluent is placed in such a way that it is able to move down slowly. The separation of the components is depend upon the degree of adsorption of components on the walls of adsorbent column. Component which has higher absorptivity is remain on the top while the other which have low absorptivity is flow down to different heights according to their values.
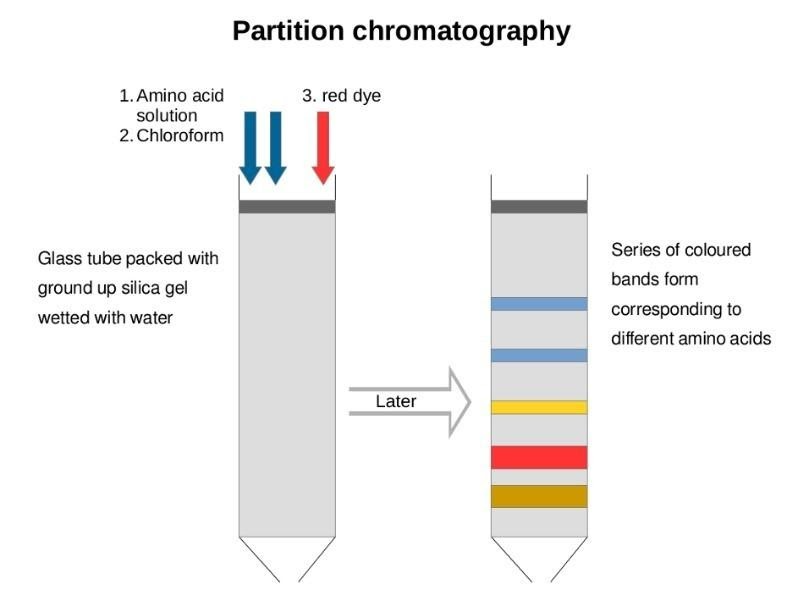
4. Partition chromatography: Partition chromatography contains a continuous different type of partitioning of various components present in a mixture which is present in stationary as well as mobile phase. The main example of partition chromatography is paper chromatography and the procedure is same as thin layer chromatography. Here chromatography paper is act as a stationary phase which further suspended in a mixture of solvents which acts as a movable i.e. mobile phase.
This type of chromatography can be shown as:
These are main types of chromatography other than this many other types are also known to us like gas chromatography, HPLC where HPLC full form is High Performance Liquid Chromatography, Ion exchange chromatography etc.
Applications of Chromatography:
There are many applications of chromatography out of which few are discussed as below:
1. The main use of chromatography is it used for the separation, isolation and purification of proteins from mixture of complex samples.
2. Used to separate out proteins from a number of compounds like lipids and nuclei acids. It is one of the most important part for the analysis and purification of proteins.
Examples of chromatography contains reversed phase chromatography, affinity chromatography, ion exchange chromatography etc.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
NCERT Chemistry Notes:
