Grignard Reagent - Structure, Preparation, Application, Uses, FAQs
Have you ever wondered how a whole molecule is formed just by starting with something as simple as an alkyl halide? The answer to this question will be known after reading this article on the Grignard reagent. Grignard reagent is an Organometallic compound written as RMgX, where R is an alkyl or aryl group and X is a halogen. Just as salt is inseparable from food, so is the Grignard reagent from Organic chemistry.
This Story also Contains
- Structure Of Grignard Reagent
- Preparation Of Grignard Reagent
- Applications Of Grignard Reagents
- Uses Of Grignard Reagent
- Some Solved Examples
A French Chemist named Francois Grignard discovered this reagent in 1900. He was investigating the organic reactions of halides with metals, and while reacting different metals with organic halides, he noticed that the reaction of magnesium with organic halides in the presence of dry ether forms a very reactive organo-magnesium compound. Due to this discovery, he was awarded the Nobel Prize in 1912. In this article, we will cover the topic of the Grignard Reagent. This topic falls under the broader category of Haloalkanes And Haloarenes, which is a crucial chapter in (Class 12 Chemistry). It is not only essential for board exams but also for competitive exams like the JEE Mains Exam , National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more
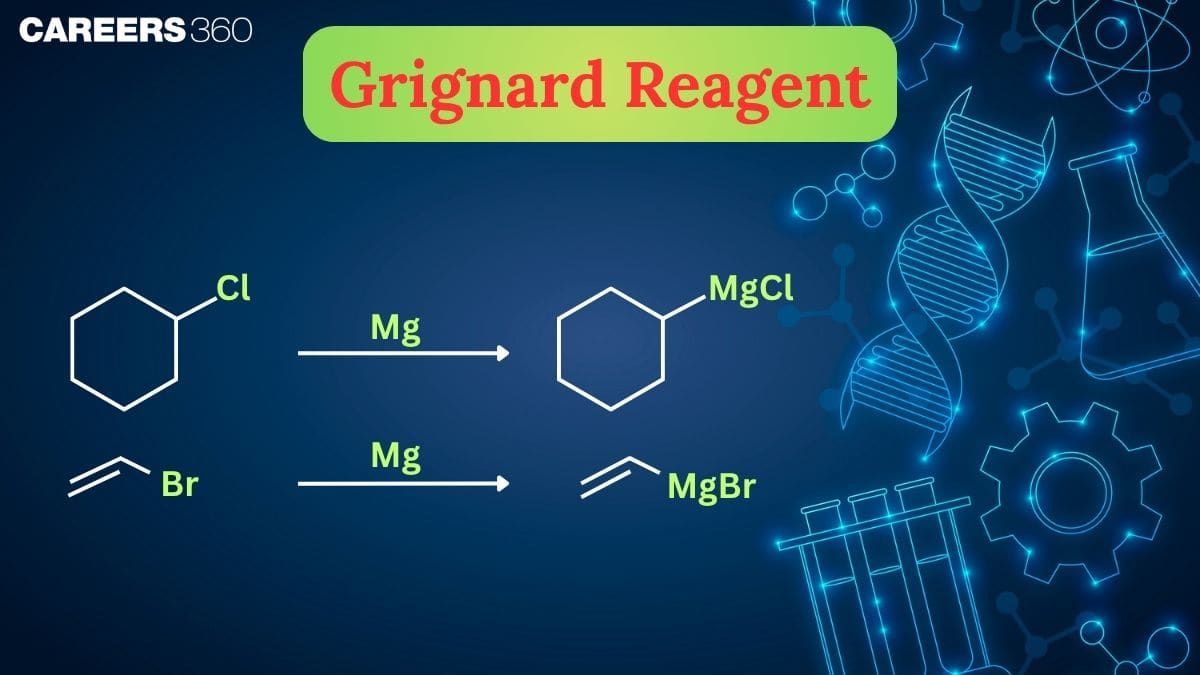
Structure Of Grignard Reagent
The structure of Grignard’s reagent involves a polar carbon-magnesium bond, with magnesium having a partially positive charge and carbon having a partially negative charge. The polarisation enables Grignard’s reagent to act as a strong nucleophile that can attack an electrophilic centre. Grignard’s reagent exists as a monomer and dimer and is stabilized by an ether molecule coordinated with the magnesium atom.
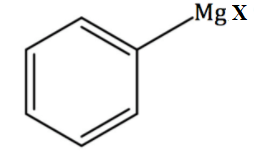
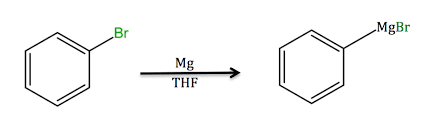
Preparation Of Grignard Reagent
Grignard reagents are prepared from magnesium metal by treating it with an organic halide. For stabilizing these organomagnesium compounds ethers are required. These compounds require air-free conditions, and the use of protic solvents may not be used as it will create protonolysis or oxidation may destroy these compounds.
However, this compound can be formed in solution by the use of ultrasound since it activates the magnesium and, thereby, consumes water present in the solution. An anhydrous condition is more suitable for the preparation of the Grignard reagent. Following the correct procedures results in the formation of the Grignard reagent. The following figure shows the preparation of the Grignard reagent from an organic halide.
Applications Of Grignard Reagents
Synthetic Application
1. Formation of Alkane
Grignard reagent, on reaction with any compound containing an active H atom, produces an alkane.
$\mathrm{MgRX}+\mathrm{HZ} \rightarrow \mathrm{MgXZ}+\mathrm{RH}$
2. Formation Of Alkene
Unsaturated halide with Grignard reagent gives alkene
$\mathrm{CH}_3 \mathrm{MgI}+\mathrm{CH}_2=\mathrm{CHI} \longrightarrow \mathrm{CH}_2=\mathrm{CHCH}_3+\mathrm{MgI}_2$
3. Formation of Alkynes
Lower alkyne $+G R \rightarrow$ Product $\xrightarrow[\text { haldide }]{\text { alks }}$ Higher alkyne
$\mathrm{R}-\mathrm{C} \equiv \mathrm{C}-\mathrm{H}+\mathrm{CH}_3 \mathrm{MgBr} \xrightarrow[-\mathrm{CH}_4]{ } \mathrm{R}-\mathrm{C} \equiv \mathrm{C}-\mathrm{MgBr}$
4. Formation of Alcohol
(a) Primary alcohol: Obtained from :
(i) Dry oxygen
(ii) Epoxy ethane
(iii) Formaldehyde
(b)Secondary Alcohol: obtained from:
(i) all aldehydes except formaldehyde
(ii) Ethyl formate + 2 moles of RMgX
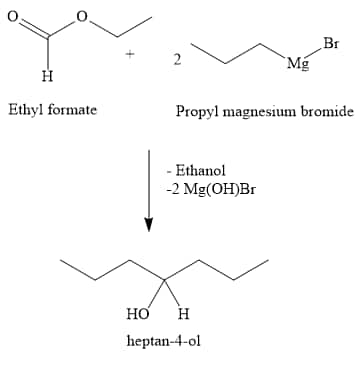
(c) Tertiary Alcohol: Obtained from:
(i) All esters except ethyl formate
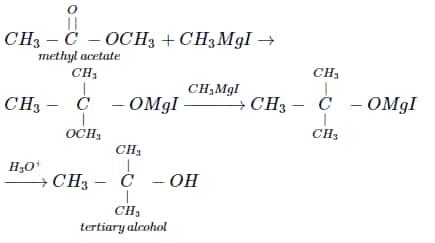
(ii) All ketones
.png)
(iii) (RCOCl) + 2 moles of RMgX
.png)
5. Formation of Aldehydes:
(i) From HCN
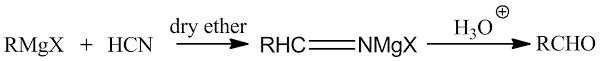
(ii) From ethyl formate + 1 mole of RMgX
.png)
6. Formation of ketones:
(i) Alkyl Cyanide
$\mathrm{CH}_3 \mathrm{CN}+\mathrm{C}_2 \mathrm{H}_5 \mathrm{MgBr} \rightarrow \mathrm{CH}_3 \mathrm{COC}_2 \mathrm{H}_5+\mathrm{NH}_3+\mathrm{MgBrOH}$
(ii) Acetyl Chloride
$\mathrm{CH}_3 \mathrm{COCl}+\mathrm{CH}_3 \mathrm{MgX} \rightarrow \mathrm{CH}_3 \mathrm{COCH}_3+\mathrm{MgXCl}$
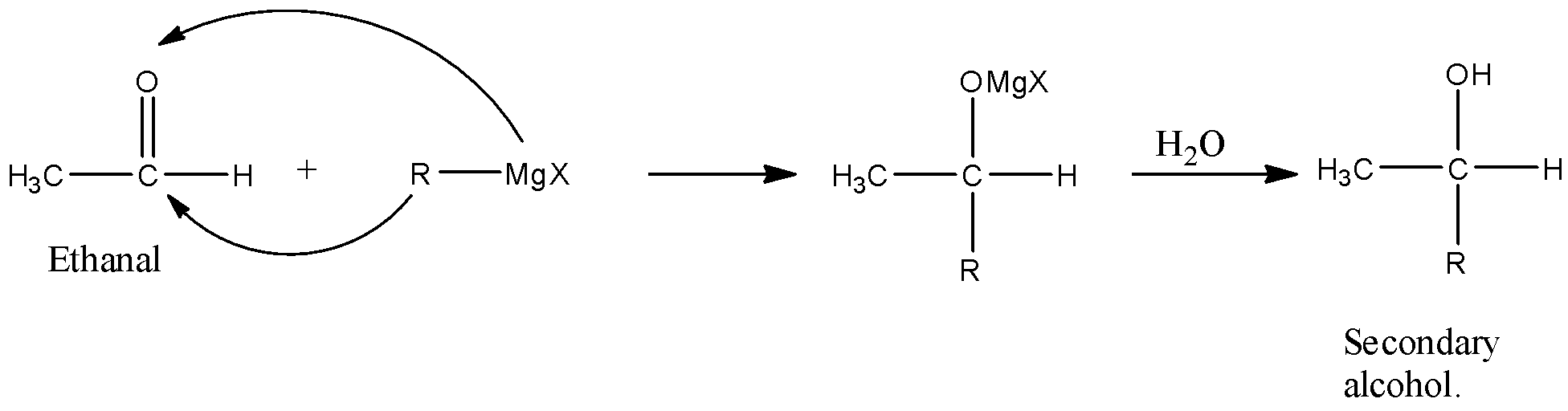
Reaction With Carbonyl Compounds
Grignard reagents react with carbonyl compounds like ketones and aldehydes to form corresponding alcohols. The nature of the substituent that gets attached to the carbonyl compound determines the product. When methanal is used as an aldehyde, the obtained alcohol will be primary, and if any aldehyde other than this is used, a secondary aldehyde is obtained. It can also be used for the alkylation of aldehydes and ketones. The Grignard reagent acts as a nucleophile, and thereby nucleophilic substitution reactions take place. The figure below shows the reaction of the Grignard reagent to form Benzyl alcohol.
Reaction Of The Grignard Reagent With Water
The reaction of a carbonyl compound with the Grignard reagent.
Grignard Reagents Acting as a Base
Grignard reagents are basic compounds and they react with phenol alcohol acceptor to give their corresponding alkoxides that is ROMgBr.
|
Related Topics Link, |
Grignard agents can be used for the alkylation of metals
Grignard reagents react with the metal to form their related compound. For example, when the Grignard reagent reacts with cadmium chloride, it forms a dialkyl cadmium by the transmethylation reaction. The following reaction explains this.
$2 \mathrm{RMgX}+\mathrm{CdCl}_2 \mathrm{R}_2 \mathrm{Cd}\rightarrow 2 \mathrm{MgXCl}$
Schlenk Equilibrium
Grignard reagent reaction with dioxane to give diorganomagnesium compounds, and the reaction involving is known as the Schlenk equilibrium. And the reaction is conducted in a solvent that is diethyl ether and tetrahydrofuran.
$2 \mathrm{RMgX}+$ dioxane $\rightarrow \mathrm{R}_2 \mathrm{Mg}+\mathrm{MgX} \mathrm{X}_2$
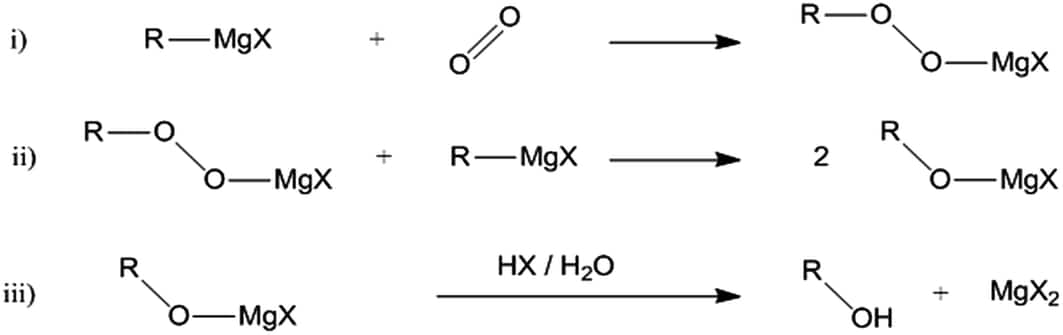
Oxidation
Grignard reagent reacts with oxygen and forms magnesium organic peroxide. The further hydrolysis of the compound obtained hydroperoxides or alcohol. The following reaction shows the formation of this, and it proceeds in radical intermediates.
Also Read:
Uses Of Grignard Reagent
- Grignard reagents are a very important organic compound that has many applications in the chemical field, mainly the organic chemistry field.
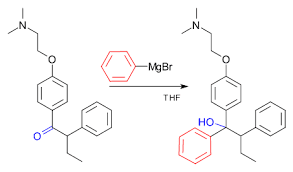
- Grignard reagents have industrial applications, also. The very important use of the Grignard reagent is the production of tamoxifen a medicine used for the treatment of breast cancer. The reaction below shows the preparation of tamoxifen.
- Grignard reagents can be used to produce alcohol from epoxides.
- Grignard reagent reaction with aldehyde, ketone, and esters to form alcoholic compounds.
- It can be used for the degradation reaction of Grignard reagents used for the chemical analysis of triacylglycerol, and also for some cross-coupling reactions that are involved in the formation of carbon-carbon and carbon-hydrogen bonds.
- It can be used for the synthesis of many organometallic compounds.
- Grignard reagents are best for the preparation of amides, acetals, amino compounds, organosulfur compounds, ethers, ketones, aldehydes, etc.
- It can be used for the production of several compounds that have a very important application in the pharmaceutical, perfume, and specialty chemicals fields.
- Grignard reagents are used for the production of optically active compounds by the reaction of a secondary alcohol with the alkyl halides in the presence of a chiral phosphine metal as a catalyst.
Also check-
Some Solved Examples
Question.1 When phenyl magnesium bromide reacts with tert. Butanol, which of the following is formed?
1)Tert. butyl methyl ether
2) (correct)Benzene
3)Tert. butyl benzene
4)Phenol
Solution
As we learned,
Zerewitinoff Method -
The reaction of the alcohol with the Grignard reagent.
- wherein
$R^{\prime} OH+RMgX \rightarrow RH+R^{\prime} OMgX$
C6H5MgBr + (CH3)3C-OH $\rightarrow$ C6H6 + [(CH3)3Co]MgBr
Hence, the answer is option (2).
Question 2 Reaction of ROH with R'MgX produces:
1)RH
2) (correct)R'H
3)R-R
4)R-R'
Solution
Alkyl magnesium halides(RMgX) are called Grignard reagents. These undergo double decomposition reactions with water, ammonia, alcohol or amine having active H atom(attached to strongly electronegative O, N, S, or F and triple bond, etc.) to give alkane corresponding to an alkyl group of Grignard reagent. The reaction occurs as follows:

So,
R'MgX+ROH$\rightarrow$R'H+Mg(OR)X
Therefore, option (2) is correct.
Question.3 Which of the following compounds will form a hydrocarbon on reaction with a Grignard reagent?
1) (correct)CH3CH2OH
2)CH3CHO
3)CH3COCH3
4)CH3CO2CH3
Solution
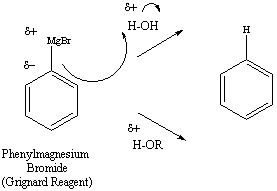
Reaction of Grignard reagent with H2O -
Alkane / Benzene is obtained
- wherein
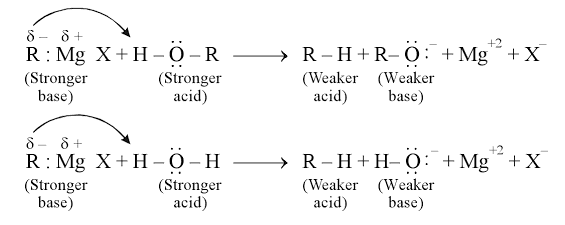
Reaction of Grignard reagent with Alcohol -
Alkane is obtained.
- wherein
Practice More Questions From The Link Given Below
For more questions to practice, the following MCQs will help in the preparation for competitive examinations
Some related topics:
Frequently Asked Questions (FAQs)
When a Grignard reagent reacts with water, it forms an alkane and magnesium hydroxide. For example:
R-Mg-X + H2O→ R-H + Mg(OH)X
An organomagnesium compound with the general formula RMgX is known as a Grignard Reagent. Where R represents organic groups such as alkyl or aryl, X represents Halogen gases, and Mg is the symbol of Magnesium.
Reaction of organic halide with magnesium in anhydrous ether leads to the formation of Grignard reagent. It is essential for the reaction to conduct in anhydrous condition because Grignard reagents react vigorously with water.
The Grignard reagent is prepared under anhydrous conditions is because of the reaction of the Grignard reagent with water. It reacts very quickly with any proton-containing compound and forms a hydrocarbon. So the effect of the Grignard reagent and its application is lost. The removal of moisture before conducting the preparation is very necessary.
R-Mg-X+H2O→R-H+Mg(OH)X
The Grignard reagent is highly reactive. There are certain precautions to consider while handling Grignard reagent:
- Must be kept away from water.
- Gloves and Goggles should be used while handling
- Reactions of the Grignard reagent must be carried out in a ventilated place
- Proper disposal
Yes, Grignard reagent reacts with many solvents, particularly those containing acidic protons such as alcohol, water, and acids.
Grignard reagents are widely used in organic chemistry :
- In the formation of alcohols by nucleophilic addition to carbonyl compounds (aldehydes and ketones).
- In the synthesis of various complex organic molecules.
- The preparation of carboxylic acids and other functional groups via reactions with carbon dioxide.
.png)
