Haloarene
Imagine that you would like to formulate a new fragrance, a perfume that smells radically different from other fragrances. You could do this by carefully combining several aromatic compounds. Similarly, the synthesis of complicated molecules often entails complicated procedures. One such process involves haloarenes, aromatic compounds in which one or more hydrogen atoms are replaced by halogen atoms. They are the building blocks of organic chemistry, and their manipulation is very important in synthesizing pharmaceuticals, agrochemicals, and polymers.
This Story also Contains
- Definitions and Explanations
- Different Aspects and Examples
- Relevance and Applications
- Some Solved Examples
- Summary
The presence of the halogen atom makes haloarenes a unique set because even though it is electron-withdrawing, it is of large size and hence able to significantly influence the chemical behavior of the aromatic ring. Of all the possible reactions involving haloarenes, the elimination-addition mechanism stands out due to its capacity to form complex structures of aromatics. This mechanism has two critical steps: elimination and addition.
Definitions and Explanations
Elimination-addition mechanism, also known as the benzyne mechanism, is a two-step process usually noticed in the chemistry of haloarenes. This generally occurs at very high temperatures or in the presence of strong bases. First, it undergoes an elimination process wherein one halogen atom is eliminated along with a hydrogen atom from the benzene ring to form a highly reactive intermediate called benzyne. This highly unstable and very short-lived intermediate is characterized by a triple bond within the aromatic ring. In the second step, the nucleophile is added to the benzyne intermediate to form a new aromatic compound. This mechanism is of paramount importance for synthetic organic chemistry because it builds complicated structures of aromatics that are otherwise tough to synthesize.
From hydrocarbons by electrophilic substitution
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts like iron or iron(III) chloride.

The ortho and para isomers can be easily separated due to large differences in their melting points. Reactions with iodine are reversible in nature and require the presence of an oxidizing agent $(\mathrm{HNO} 3, \mathrm{HIO} 4)$to oxidize the HI formed during iodination. Fluoro compounds are not prepared by this method due to the high reactivity of fluorine.
From amines by Sandmeyer’s reaction
When a primary aromatic amine, dissolved or suspended in cold aqueous mineral acid, is treated with sodium nitrite, a diazonium salt is formed. Mixing the solution of freshly prepared diazonium salt with cuprous chloride or cuprous bromide results in the replacement of the diazonium group by –-Cl or -Br.


Different Aspects and Examples
The mechanism of elimination-addition varies with conditions and reactants. One of the prominent examples is the reaction of chlorobenzene with a strong base, such as sodium amide (NaNH2) in liquid ammonia. In the case of chlorobenzene, an elimination takes place with the formation of benzyne, which gets attacked by the amide ion and forms aniline. Another example is the reaction of bromobenzene with potassium tert-butoxide KOtBu, leading to the formation of benzyne, which afterward reacts with tert-butanol and forms tert-butylphenol. These reactions demonstrate the versatility of the elimination-addition mechanism in introducing a wide variety of functional groups into the aromatic ring. Also, the benzyne intermediate can take part in Diels-Alder reactions, which makes this mechanism further synthetically useful.
The accepted mechanism for nucleophilic aromatic substitution in nitro-substituted aryl halides is given as follows.

Mechanism
- Addition stage: The nucleophile, in this case, methoxide ion, adds to the carbon atom that bears the leaving group to give a cyclohexadienyl anion intermediate.

- Elimination stage: Loss of halide from the cyclohexadienyl intermediate restores the aromaticity of the ring and gives the product of nucleophilic aromatic substitution.

Relevance and Applications
The elimination-addition mechanism is not some kind of chemical curiosity; on the other hand, it is of important practical application. This mechanism is applied in pharmaceuticals to synthesize complex molecules containing some functional groups responsible for their biological activity. For example: some anti-cancer drugs and antibiotics are prepared by this approach. In agrochemicals, haloarenes are used for the production of herbicides and pesticides with high efficiency and selectivity. Mechanistically, it provides insight into the kinetics of reaction and behavior of such reactive intermediate involved that is really enlightening. The mechanism of elimination-addition for aromatic compounds allows manipulation to tailor-make material properties of new materials; this, for example, is very important in industry for, say, polymers and dyes. What makes benzyne an uncommonly useful tool in the synthetic chemist's armamentarium is the unusual reactivity of the intermediate, able to stitch together complex molecular architectures.
Recommended topic video on(haloarenes)
Some Solved Examples
Example 1
Question:
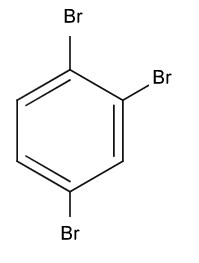
Identify Z in the given reaction sequence

1) (correct)

2)

3)
![]()
4)

Solution:
As we have learnt,
Sandmeyer’s reaction
When a primary aromatic amine, dissolved or suspended in cold aqueous mineral acid, is treated with sodium nitrite, a diazonium salt is formed. Mixing the solution of freshly prepared diazonium salt with cuprous chloride or cuprous bromide results in the replacement of the diazonium group and leads to the formation of the respective aromatic halide.

Therefore, option (1) is correct.
Example 2
Question:
X reacts with $\mathrm{Cl}_2 / \mathrm{FeCl}_3$ and gives chlorobenzene.What is X in this reaction
1) Benzene
2)Phenol
3)Toluene
4)Ethyl benzene
Solution:
The reaction occurs as
Chlorination of Benzene in the presence of Lewis acid forms chlorobenzene.
So, X is benzene.
Therefore, option (1) is correct.
Example 3
Question:
Consider the following reactions:
(a)
(b)
(c)
(d)
Which of these reactions are possible?
1) a and d
2) b and d (correct)
3) b, a and d
4) a and d
Solution:
As we have learned,
1. Aryl halides (a) do not give Friedel Craft's reaction with Benzene because the unstable Phenyl carbocation is very difficult to obtain.
2. Vinyl halides (c) do not give Friedel Craft's reaction because the unstable vinyl carbocation is very difficult to obtain.
Reactions given in (b) and (d) are correct.
Therefore, Option(2) is correct.
Summary
The mechanism thus represents one very central elimination-addition process in organic chemistry, which enables the synthesis of a very broad and diversified range of aromatic compounds. It is this mechanism through which a wide breadth of functionalities will be inserted into an aromatic ring via the formation and consumption of the reactive benzyne intermediate. We see the power and utility of this mechanism in examples such as the reactions of chlorobenzene and bromobenzene. Applications in pharmaceuticals, agrochemicals, and materials science further reiterate its importance for industry and academia alike.




