Haloalkanes and Haloarenes - Notes, Topics, Formula, Books, FAQs
Why do some compounds easily form carbocations or free radicals, such as PVC, anesthetics, and refrigerants? The answer to this question is halogen-substituted hydrocarbons, which are Haloalkanes and Haloarenes. From refrigerants in air conditioners to medicines, Haloalkanes and Haloarenes are everywhere around us. These words are made up of two words: the first is ‘Halo’, which means Halogens, and the second word is ‘alkanes’ or ‘arenes’, which means aliphatic or aromatic hydrocarbons.
This Story also Contains
- Important Topics - Organic Compounds Containing Halogens
- Overview of the Chapter
- Haloalkanes and haloarenes can be classified mainly on the basis of two aspects:
- Physical Properties
- Preparation of Alkyl Halides
- Chemical Reactions
- Applications of Haloalkanes and Haloarenes
- Polyhalogen Compounds
- Study Links
- Haloalkanes and Haloarenes in Different Exams
- Previous Year Questions of Haloalkanes and Haloarenes
- How to Prepare for Haloalkanes and Haloarenes
- Prescribed Books
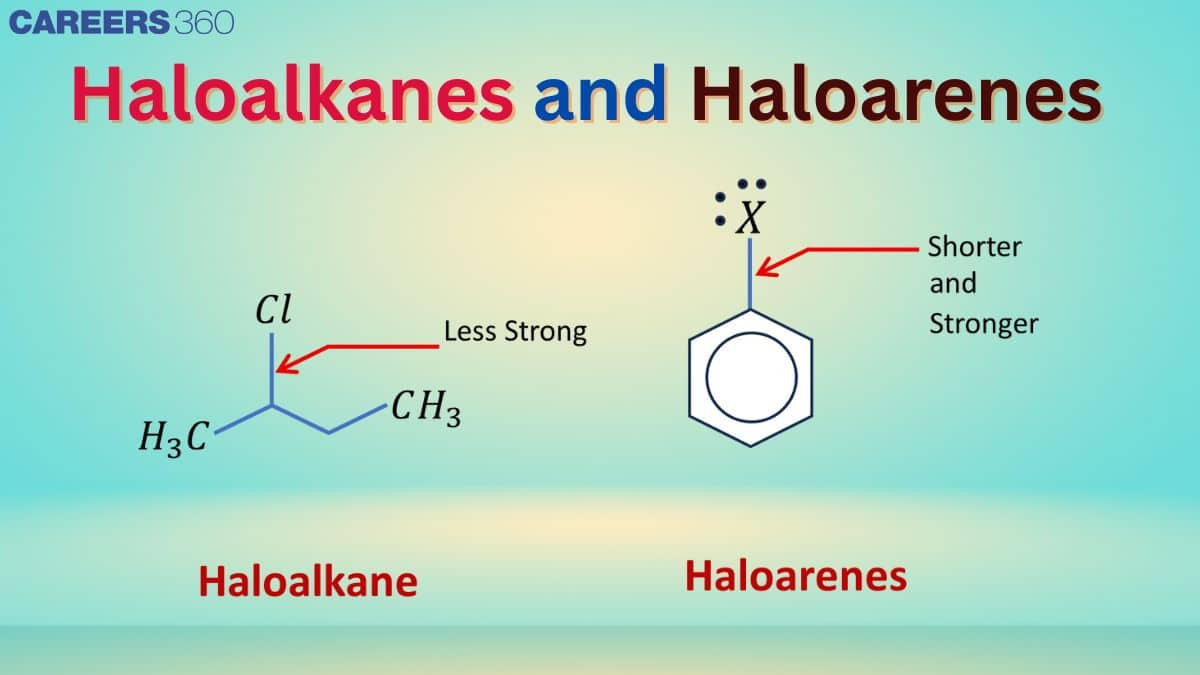
The organic compounds containing Halogens (Fluorine, Chlorine, Bromine, Iodine). These compounds play a crucial role in pharmaceuticals and industries. In these compounds, one or more Hydrogen atoms are replaced by Halogens. Many organic compounds containing halogens occur naturally and have clinical significance. Some of the widely used Haloalkanes are Chloroform ($\mathrm{CHCl}_3$) and Carbon tetrachloride ($\mathrm{CCl}_4$), and Haloarenes are DDT, Bromobenzene, etc.
Important Topics - Organic Compounds Containing Halogens
This chapter of Chemistry focuses on the structure, properties, and reactions of haloalkanes and haloarenes, with special emphasis on reaction mechanisms and practical applications.
Alkyl Halides
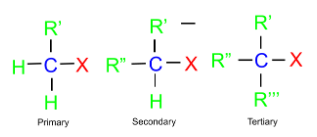
Alkyl halides are organic compounds containing a halogen atom (F, Cl, Br, or I) bonded to an alkyl group. Alkyl halides are classified as primary, secondary, or tertiary based on the carbon atom attached to the halogen. Alkyl halides are versatile intermediates in organic synthesis, used in the production of polymers, refrigerants, and agrochemicals.
Physical & Chemical Properties of Haloalkanes
Haloalkanes exhibit unique physical properties like higher boiling points compared to alkanes due to the polar C-X bond and their larger molecular mass. Chemically, they undergo nucleophilic substitution ($S_N{ }^1$ and $S_N{ }^2$ mechanisms) and elimination reactions. The reactivity depends on the halogen and the carbon skeleton.
Haloarenes
Haloarenes are aromatic compounds where one or more hydrogen atoms in an aromatic ring are replaced by halogen atoms. They are less reactive than haloalkanes due to resonance in the benzene ring, which delocalizes the electrons and stabilises the C-X bond. Haloarenes find applications in pesticides, dyes, and pharmaceuticals.
Markovnikov Rule
Markovnikov's Rule predicts the outcome of electrophilic addition reactions to alkenes. It states that when a protic acid (HX) adds to an asymmetric alkene, the hydrogen atom attaches to the carbon with more hydrogen atoms, and the halide (or other substituent) attaches to the carbon with fewer hydrogen atoms. This rule is explained by the stability of the carbocation intermediate, which favors the more substituted and stable carbocation.
Example
Addition of HBr to propene:
$\mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}_2+\mathrm{HBr} \rightarrow \mathrm{CH}_3-\mathrm{CHBr}-\mathrm{CH}_3$
Major product: 2-Bromopropane
Also read,
Overview of the Chapter
Haloalkanes and haloarenes can be classified mainly on the basis of two aspects:
- On the basis of the number of halogen atoms
Halogens may be classified as mono, di or polyhalogen compounds depending on the number of halogen compounds attached to the carbon compound.
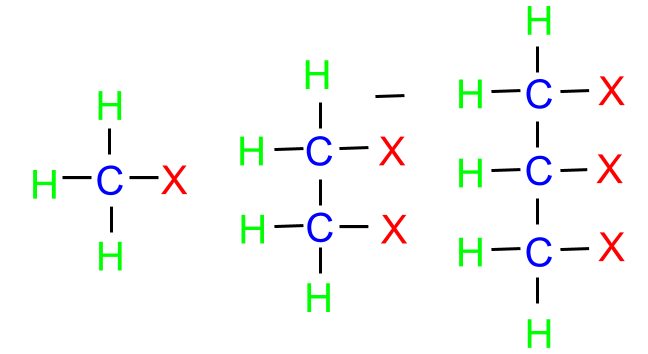
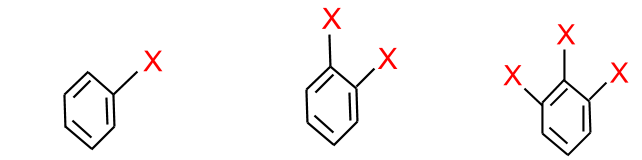
- Halogen attached to sp3 or sp2 carbon
(a) Alkyl Halide: In this class, the halogen atom is bonded to the sp3 hybridised carbon. These can further be classified into three categories as follows:
(b) Allylic Halides: In this class, the halogen atom is attached to the $\mathrm{sp}^3$ hybridised carbon, which is attached to the carbon-carbon double bond.
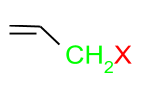
(c) Benzylic halides: These are the halides in which the halogen atom is attached to $\mathrm{sp}^3$ hybridised carbon, which is attached to an aromatic ring.
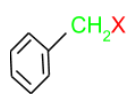
(d) Vinylic Halides: In this type of halide, the halogen atom is attached to $\mathrm{sp}^2$ carbon.
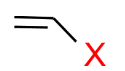
(e) Aryl Halides: In this type of halide, the halogen atom is $\mathrm{sp}^2$ carbon atom of an aromatic ring.
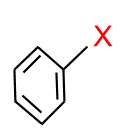
Physical Properties
- Melting and boiling points: The melting and boiling points of haloalkanes and haloarenes are determined by the intermolecular forces of attraction between them. The boiling point of alkyl halides follows this order:
$\mathrm{R}-\mathrm{I}>\mathrm{R}-\mathrm{Br}>\mathrm{R}-\mathrm{Cl}>\mathrm{R}-\mathrm{F}$
The boiling point is always determined by van der Waals forces. As the molecular size increases, the boiling point increases. For example, in the case given below:
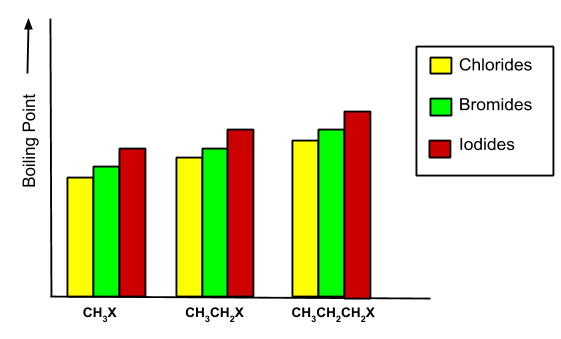
As branching increases in alkyl halides, the boiling point decreases. - Density: The density increases as the size of the haloalkanes and haloarenes increases. It also increases with the increase of the atomic mass of halogen.
Preparation of Alkyl Halides
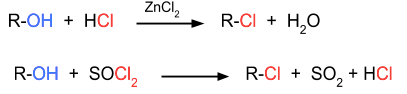
- Alkyl halides are prepared in many ways. The most common method to prepare alkyl halides is from alcohols. In this method, the alcohols are reacted with halogen acids, thionyl chloride or phosphorus halides, then the hydroxyl group is replaced with a halogen group.
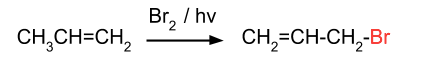
- From alkene: Alkyl halide can be prepared from alkenes by reaction with hydrogen halides or Br2/hv.
There are other important methods also available for the preparation of alkyl halides, such as free radical halogenation and Sandmeyer's reaction.
Chemical Reactions
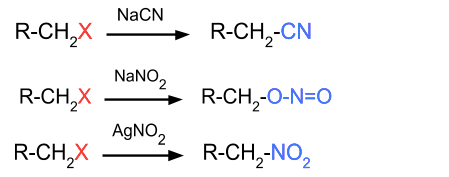
- Reaction with $\mathrm{NaCN}, \mathrm{AgCN}, \mathrm{NaNO}_2, \mathrm{AgNO}_2$: In this reaction, the halide group is replaced by CN or NO2. If an alkyl halide is reacting with NaNO2 then ONO replaces the halide group, but if AgNO2 is reacting, then NO2 replaces the halide group.
- Finkelstein Reaction: This reaction is used for the preparation of an alkyl halide from another alkyl halide. In this reaction, an alkyl halide is treated with a metal halide salt in the presence of acetone.
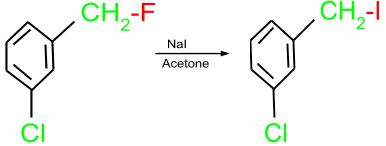
- Substitution Reactions:
Alkyl halides undergo substitution reactions either via $S_N{ }^1$ mechanism or $S_N{ }^2$ mechanism.
(a) $S_N{ }^1$ reaction: This mechanism is favourable when the tertiary alkyl halide is present. This mechanism occurs in two steps, viz, breaking of the halide bond and then the formation of the carbocation.
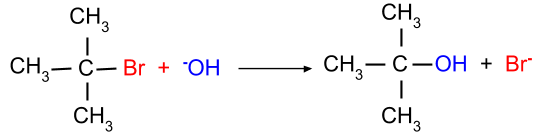
(b) $S_N{ }^2$ reaction: This reaction is favourable when the primary alkyl halide is present. In this mechanism, the breaking of the halide bond and the formation of the carbon-OH bond both occur at the same time. Since this reaction mechanism is bimolecular thus it is known as the SN2 mechanism.
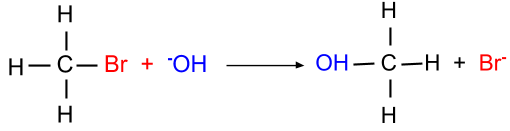
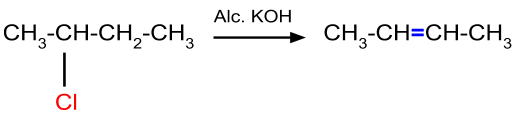
- Elimination reaction:
This reaction occurs when the alkyl halide having a β-hydrogen atom reacts with alcoholic potassium hydroxide. An example of this reaction mechanism is given below. When there is a possibility of the formation of more than one alkene, then the most stable alkene is the major product.
- Friedel-Crafts reactions:
These are those reactions in which an aromatic halide is treated with an alkyl halide or an acyl halide to attach substituents. These reactions are of two types i.e, alkylation and acylation. Both these reactions follow the electrophilic aromatic substitution mechanism. In both these reactions, the most stable product is the major product.
(a) Friedel-Crafts alkylation: In this reaction, an aromatic halide reacts with an alkyl halide and forms the product as given below in the reaction.
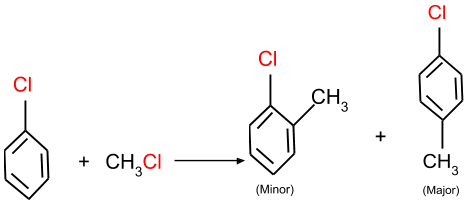
(b) Friedel-Crafts acylation: In this reaction, the aromatic halide reacts with an acyl group and forms the product as given below.
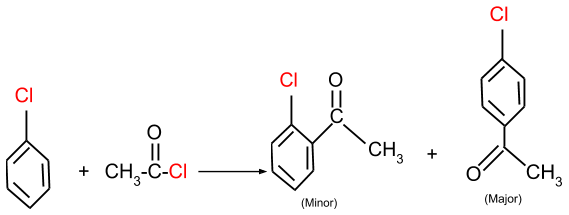
Applications of Haloalkanes and Haloarenes
There are some important applications of haloalkanes and haloarenes, viz:
- Some haloalkanes are used as synthetic intermediate compounds in labs.
- They are used in fire extinguishers.
- The derivatives of these compounds are used for medicinal purposes.
- Some naturally occurring haloalkanes act as protective agents against ocean attackers.
- They are used as propellants.
Polyhalogen Compounds
Carbon compounds containing more than one halogen atom are usually referred to as polyhalogen compounds. Many of these compounds are useful in industry and agriculture.
1. Dichloromethane (Methylene chloride)
It is widely used as a solvent in paint removers, aerosols, drug manufacturing, and metal cleaning. However, it is harmful to the central nervous system. Low-level exposure may impair hearing and vision, while higher levels can cause dizziness, nausea, and numbness. Direct skin contact leads to burning and redness, and eye contact can damage the cornea.
2. Trichloro methane (Chloroform)
It is mainly used as a solvent and in making refrigerant R-22. Once used as an anaesthetic, it is now avoided due to its toxicity. Inhalation can cause dizziness and fatigue; long-term exposure may harm the liver and kidneys.
$2 \mathrm{CHCl}_3+\mathrm{O}_2 \xrightarrow{\text { light }} 2 \mathrm{COCl}_2+2 \mathrm{HCl}$
3. Triiodomethane (Iodoform)
It was used earlier as an antiseptic but the antiseptic properties are due to the liberation of free iodine and not due to iodoform itself. Due to its objectionable smell, it has been replaced by other formulations containing iodine
4. Tetrachloromethane (Carbon tetrachloride)
It is used in making refrigerants, solvents, and chemicals. Once common as a cleaner, it is now restricted due to its toxicity. Exposure can cause nausea, nerve damage, heart issues, and liver cancer. It also depletes the ozone layer, increasing the risk of skin cancer and other health problems.
5. Freons
They are chlorofluorocarbon compounds of methane and ethane, widely used as aerosol propellants and in refrigeration and air conditioning. Freon-12, made from tetrachloromethane via the Swarts reaction, is the most common. These stable, non-toxic gases eventually reach the stratosphere, where they trigger radical reactions that deplete the ozone layer.
6. p,p’-Dichlorodiphenyltrichloroethane(DDT)
DDT, the first chlorinated organic insecticide. The use of DDT increased enormously on a worldwide basis after World War II, primarily because of its effectiveness against the mosquito that spreads malaria and the lice that carry typhus. However, problems related to the extensive use of DDT began to appear in the late 1940s. Many species of insects developed resistance to DDT, and it was also discovered to have a high toxicity towards fish. The chemical stability of DDT and its fat solubility compounded the problem. DDT is not metabolised very rapidly by animals; instead, it is deposited and stored in the fatty tissues.
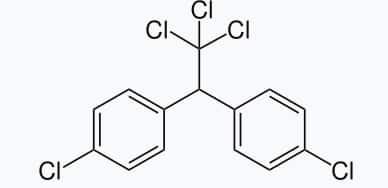
Study Links
Given below some links to access the important resources for better understanding of the chapter:
Haloalkanes and Haloarenes in Different Exams
This chapter is commonly asked in board and competitive examinations with focus on reaction mechanisms, nomenclature, and comparative reactivity of organic halogen compounds:
| Exam Name | Focus Area | Common Topics Asked | Preperation Tips |
| CBSE Board | Conceptual understanding | Nomenclature, reactions of haloalkanes | Revise NCERT reactions and mechanisms |
| JEE Main | Concept application | SN1 & SN2 reactions, reactivity order | Practise mechanism-based MCQs |
| JEE Advanced | Analytical & mechanism-based | Reaction mechanisms, neighbouring group effect | Focus on detailed mechanisms |
| NEET | Basic concepts | Uses, reactions, IUPAC naming | Study NCERT line by line |
| State Board Exams | Theory-oriented | Preparation methods, properties | Learn definitions and reactions |
| Chemistry Olympiads | Advanced application | Reaction mechanisms, stereochemistry | Practise advanced organic problems |
Previous Year Questions of Haloalkanes and Haloarenes
Questions from this chapter frequently appear in board and competitive exams. Given below some important questions from this chapter:
Question 1: Given below are two statements :
Statement (I): $S_N{ }^2$ reactions are 'stereospecific', indicating that they result in the formation of only one stereoisomer as the product.
Statement (II): $S_N{ }^1$ reactions generally result in the formation of products as a racemic mixture. In light of the above statements, choose the correct answer from the options given below :
(1) Statement I is true, but Statement II is false
(2) Statement I is false, but Statement II is true
(3) Both Statement I and Statement II are true
(4) Both Statement I and Statement II are false
Answer:
$S_N{ }^2$$\longrightarrow$ Inversion
$S_N{ }^1$$\longrightarrow$ Racemisation
Hence, the answer is option (3).
Question 2: Given below are two statements :
Statement (I): Alcohols are formed when alkyl chlorides are treated with aqueous potassium hydroxide by elimination reaction.
Statement (II): In alcoholic potassium hydroxide, alkyl chlorides form alkenes by abstracting the hydrogen from the β-carbon.
In light of the above statements, choose the most appropriate answer from the options given below :
(1) Both Statement I and Statement II are incorrect
(2) Statement I is incorrect, but Statement II is correct
(3) Statement I is correct, but Statement II is incorrect
(4) Both Statement I and Statement II are correct
Answer:
Statement (I) :
R−Cl$\xrightarrow{\mathrm{aq KOH}}$R−OH. This reaction is nucleophilic substitution ($S_N{ }^2$ or $S_N{ }^1$), not elimination.
Statement (II) :
.png)
Hence, the correct answer is option (2).
Question 3: Give reasons for the following observation :
p-Chloronitrobenzene reacts with (aq) NaOH at 443 K to give p-nitrophenol, whereas chlorobenzene reacts with the same reagent at 623 K and 300 atm.
Solution:
1. p-Chloronitrobenzene reacts with (aq) NaOH at 443 K to give p-nitrophenol
p-chloronitrobenzene undergoes nucleophilic substitution faster than chlorobenzene because p-chloronitrobenzene has a nitro group which is an electron-withdrawing group and chlorine itself is an electronegative group by which chlorine generates electrons on its surface and attract as well as .it also generate partial positive charge therefore now it become an electrophile and it require electron for that positive charge than the nitro group withdraw its electron and increase the partial positive charge over carbon and chloro group and weaken their bond it goes easily on nucleophilic substitution reaction than chlorobenzene.

2. Chlorobenzene reacts with aqueous NaOH at 623 K and 300 atm
Because chlorobenzene lacks strong electron-withdrawing groups that stabilise the intermediate, that's why the reaction requires harsh conditions.

Question 4: Which one of the following reactions will not lead to the desired ether formation in a major proportion? (iso- $\mathrm{Bu} \Rightarrow$ isobutyl, sec- $\mathrm{Bu} \Rightarrow$ sec-butyl, $\mathrm{nPr} \Rightarrow$ n-propyl, ${ }^{\text {' } \mathrm{Bu}} \Rightarrow$ tert-butyl, $\mathrm{Et} \Rightarrow$ ethyl)
1) ${ }^{\prime} \mathrm{BuO} \stackrel{\ominus}{\mathrm{N}} \mathrm{a}+\mathrm{EtBr} \rightarrow{ }^{\mathrm{t}} \mathrm{Bu}-\mathrm{O}-\mathrm{Et}$
2)
3)
4) iso- $\mathrm{BuO} \stackrel{\ominus}{\mathrm{N}} \mathrm{a}+\mathrm{sec}-\mathrm{BuBr} \rightarrow \mathrm{Sec}-\mathrm{Bu}-\mathrm{O}-\mathrm{iso}-\mathrm{Bu}$
Solution:
For $2^{\circ} \mathrm{RX} \Rightarrow$ elimination would be more favourable than substitution
The primary alkyl halide undergoes Williamson's synthesis for the formation of an ether
$\mathrm{RO}^{\ominus} \mathrm{Na}^{+}+\mathrm{RX} \rightarrow \mathrm{ROR}$ (undergo $\mathrm{S}_{\mathrm{N}} 2$ reaction)
(where RX = Primary alkyl halide)
Hence, the correct answer is option (4).
Practice more questions from the link given below
For more questions to practice, the following MCQs will help in the preparation for competitive examinations
How to Prepare for Haloalkanes and Haloarenes
- This chapter is part of organic chemistry and possesses a very high weightage of marks in board exams and other competitive exams like JEE and NEET. It is completely theory-based. You are not supposed to memorise any formula, but the reaction mechanisms and named reactions are very important.
- For preparing this chapter, firstly, you must have a basic understanding of the chapter "Organic Chemistry- Some Basic Principles and Techniques". After this, you must read this chapter, "Haloalkanes and Haloarenes" from NCERT thoroughly, with every concept grasped completely on your own.
- Practice lots of examples related to this chapter, because then you will be able to understand the most basic concepts related to the whole of organic chemistry.
Prescribed Books
For this chapter, first, the NCERT book is best for initial level preparation as well as for board exams. Now, after this, if you want to prepare for competitive exams like JEE and NEET, then these are the best books for you: Morrison and Boyd, and R.K. Gupta by Arihant Publication. Meanwhile, in preparation, you must continuously give mock tests to assess the depth of knowledge. Our platform will help you to provide a variety of questions for deeper knowledge with the help of videos, articles, and mock tests.
Also read,
Frequently Asked Questions (FAQs)
The key difference lies in the carbon atom to which the halogen is attached:
- In haloalkanes, the halogen atom is attached to an sp³-hybridized carbon atom of an alkyl group.
- In haloarenes, the halogen atom is directly attached to an sp²-hybridized carbon atom of an aromatic ring. This difference in hybridization and the presence of the aromatic ring significantly impact their chemical properties and reactivity.
The molecular formula of a haloalkane is generally written as R–X, where R is an alkyl group and X is a halogen. Common examples are CH₃Cl (chloromethane) and CH₃CH₂Br (ethyl bromide). For haloarenes, the formula is Ar–X, where Ar is an aryl group such as C₆H₅Cl (chlorobenzene)
Haloalkanes, also known as alkyl halides, are organic compounds that contain at least one halogen atom bonded to an alkane carbon atom. They can be classified into primary, secondary, and tertiary haloalkanes based on the degree of substitution of the carbon atom attached to the halogen.
Haloarenes are compounds where the halogen atom is attached to an aromatic ring, such as benzene. In contrast, haloalkanes have the halogen attached to a saturated carbon chain. This structural difference can lead to distinct physical and chemical properties, including reactivity and boiling points.
Haloalkanes are widely used in the chemical industry and household applications. They can serve as solvents, refrigerants, and intermediate compounds in the synthesis of pharmaceuticals, agrochemicals, and other organic compounds. Their ability to interact with various functional groups makes them versatile in chemical reactions.
Many haloalkanes are toxic and can contribute to environmental issues, especially when they are volatile or persist in the environment. Some members of this class, such as chlorofluorocarbons (CFCs), can deplete the ozone layer, while others may be harmful to aquatic life or human health if released into the environment.
When handling haloalkanes, it's important to work in a well-ventilated area or under a fume hood to avoid inhalation of fumes. Personal protective equipment, such as gloves and goggles, should also be worn to prevent skin and eye exposure, as many haloalkanes can be irritants or harmful.



