Ionic Bond or Electrovalent Bond - Definition, Examples, Properties, Principal, FAQs
Ionic, or electrovalent, bonds are part of the basics of chemistry. These bonds are formed by electrostatic attraction between ions, which means an atom or molecule has either gained or lost an electron to give it a net electrical charge. Generally, ionic bonds form between metals and nonmetals. Because metals' electronegativity is always less, they can easily lose their electrons and become positively charged cations.
This Story also Contains
- Understanding Ionic Bonds
- The Formation of Ionic Compounds
- Factors Which Favours the Formation of Ionic Bond
- Types and Features of Ionic Bonds
- Ionic Bonds and Their Applications in Real Life
- Some Solved Examples
- Practice More Questions From the Link Given Below:
In the formation of ionic bond, cations are generally formed from metallic elements by loss of electrons from their respective atoms. The ammonium ion (NH4+) is an exception. The process of loss of electrons by metal atom requires energy equal to the ionization enthalpy. Lesser the value of ionization enthalpy, greater is the tendency of the atom to form cation. For example, alkali metals form cations quite easily because of the low values of ionization enthalpies.
In this article, we will cover the concept of the stability of filled and half-filled subshells. This concept falls under the broader category of Chemical Bonding, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
Also Read:
Understanding Ionic Bonds
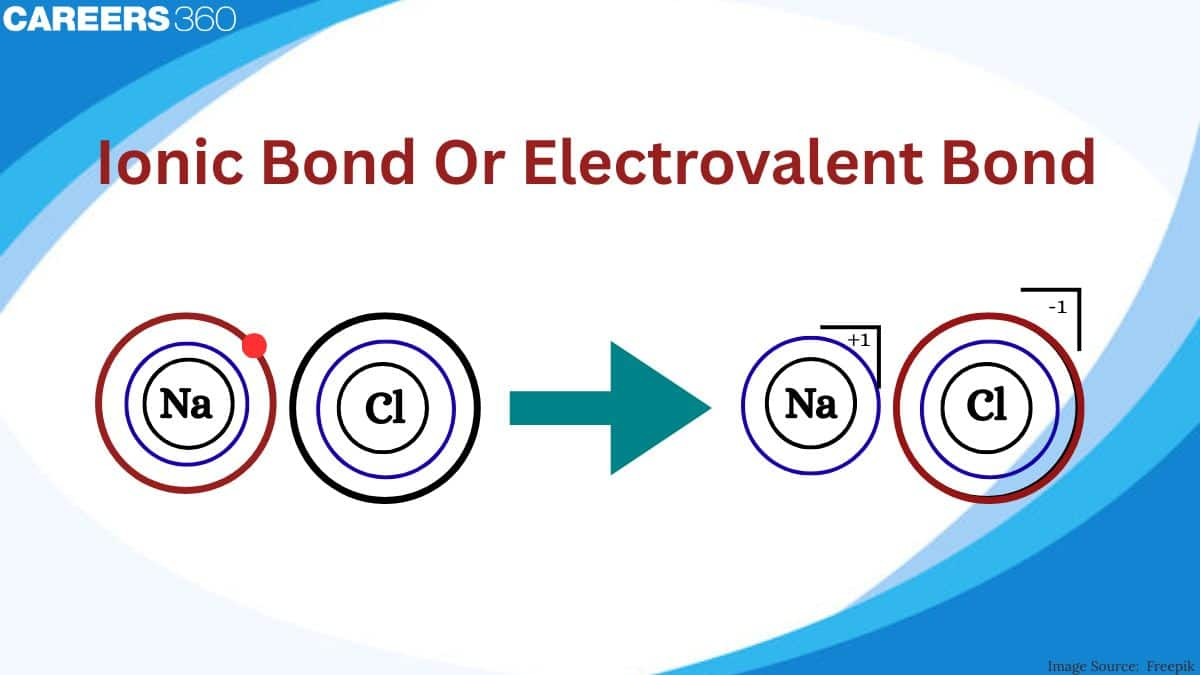
Atoms form ionic bonds through electron transfer to other atoms, developing charged ions in the process. For example, when the electron is lost by a metal atom, like sodium, Na, it becomes a positively charged ion, Na⁺. On the other hand, when a nonmetal atom, let's say chlorine, Cl, gains an electron, the atom it forms becomes a negatively charged ion, Cl⁻. This strong electrostatic force of attraction between these oppositely charged ions leads to the forming of an ionic bond and results in an ionic compound like common salt, which is sodium chloride or NaCl
Ions are atoms or molecules bearing an electrical charge. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds or electrostatic forces of attraction between oppositely charged cations and anions. Ionic solids exhibit a crystalline structure and tend to be rigid and brittle; they also have high melting and boiling points, which suggests that ionic bonds are very strong. Ionic solids are poor conductors of electricity as the strength of ionic bonds is very strong and it prevents the ions from moving freely in the solid state. Most ionic solids, however, dissolve readily in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because, in the liquid state, these ions can move freely.
Related Topics Link:
The Formation of Ionic Compounds
Binary ionic compounds are composed just of two elements i.e., a metal (which forms the cations) and a nonmetal (which forms the anions). For example, NaCl is a binary ionic compound. Many metallic elements have relatively low ionization potentials and lose electrons easily. These elements lie to the left in a period or near the bottom of a group on the periodic table. Nonmetal atoms have relatively high electron affinities and thus readily gain electrons lost by metal atoms, thereby filling their valence shells. Nonmetallic elements are found in the upper-right corner of the periodic table.
It is important to consider that the formula for an ionic compound does not represent the physical arrangement of its ions. For example, sodium chloride (NaCl) “molecule”, because there is not a single ionic bond between any particular pair of sodium and chloride ions. The attractive forces between ions are isotropic i.e., the same in all directions in other words, any particular ion is equally attracted to all of the nearby ions of opposite charge. This results in the ions arranging themselves into a tightly bound, three-dimensional lattice structure. Sodium chloride, for example, consists of a regular arrangement of equal numbers of Na+ cations and Cl– anions as shown in the figure.

The strong electrostatic force of attraction between Na+ and Cl– ions holds them tightly together in solid NaCl. It requires 769 kJ of energy to dissociate one mole of solid NaCl into separate gaseous Na+ and Cl– ions:
NaCl(s) $\longrightarrow$ Na+(g)+Cl- $\Delta $H=769 kJ
Also Read:
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Factors Which Favours the Formation of Ionic Bond
1. Ionization enthalpy
2. Electron gain enthalpy:
Electron gain enthalpy (or electron affinity) is the energy released when gaseous atom forms a negative ion. Thus, the value of electron gain enthalpy gives the tendency of an atom to form anion. Greater the negative value of electron gain enthalpy, more is the tendency of an atom to form anion. For example, Halogens have largest negative values of electron gain enthalpies within their respective period, hence, they form ionic compounds with metals very easily.
3. Lattice enthalpy: The lattice enthalpy of an ionic solid is defined as the energy required to completely seperate one mole of a solid ionic compound into gaseous constituent ions. For example, the lattice enthalpy of NaCl is 788 kj mol-1. This means that 788 kj of energy is required to seperate one mole of solid NaCl into one mole of Na+ (g) and one mole of Cl- (g) to an infinite distance.
.jpg)
Types and Features of Ionic Bonds
Ions can also be classified according to the nature of the elements taking part in the bond. They are mainly formed between metals and nonmetals. The metal loses electrons, and the nonmetal gets them. A very fine example of an ionic bond is that formed between sodium and chlorine to form NaCl.
The main features of ionic bonds are:
1. Ion Formation: Metals lose electrons to form cations; nonmetals gain electrons to form anions.
2. High MP and BP: The melting and boiling points of ionic compounds are generally high because of the strong ionic attractions involved.
3. Electrical Conductivity: A certain conductivity results when ionic compounds are melted or dissolved in water, as a result of the free movement of the ions.
4. Brittleness: Ionic compounds are, for the most part, brittle and shatter upon application of stress.
All these properties underline the importance of ionic bonds in chemical processes and the applications of those in various spheres.
Also Read :
Ionic Bonds and Their Applications in Real Life
Ionic bonds are very important both in everyday life and in many branches of science. Table salt, or sodium chloride, is an ionic compound that is widely used in the culinary arts as flavoring in foods and as a preservative. Many fertilizers, such as ammonium nitrate, are ionic compounds that provide plants with critical nutrients they need to grow.
In materials science, the ionic bond forms ceramics and glass—basic constituents of construction and manufacturing. Ionic compounds play a lead role in electronics; for instance, in a lithium-ion battery, it is even through ionic interactions that energy is stored and passed.
Recommended topic video on (Ionic Bond or Electrovalent Bond )
Some Solved Examples
Example 1:
Which of the following compounds contain(s) no covalent bond(s) ?
KCl, PH3, O2, B2H6, H2SO4
1) KCl, B2H6,
2) KCl, B2H6, PH3,
3) KCl, H2SO4
4) KCl
Solution:
As we learned in the concept
Ionic Bonding -
The formation of an ionic bond takes place between a metal and a non-metal by the transfer of electrons.
- wherein
e.g $\mathrm{NaCl}, \mathrm{CaCl}_2$ etc.
$\mathrm{KCl} \Rightarrow$Ionic compound
Hence, the answer is an option (4).
Example 2:
The atomic number of four elementsP,Q,R,S are6,8,10 and 12 respectively.
The two elements which can react to form ionic compounds are:
1) $P$ and $S$
2) $Q$ and $R$
3) $P$ and $R$
4) $Q$ and $S$
Solution:
Q atom’s electronic configuration is 2,6, and the S atom is 2,8,2. Therefore, S has 2 electrons in its outermost shell so that it will lose 2 electrons to Q, and both will attain inert gas configuration.
Hence, the answer is the option (4).
Example 3:
What is the electronic configuration of calcium ion (Ca2+)
1) 2,8
2) 2,8,2
3) 2,8,4
4) 2,8,8
Solution:
The atomic number of calcium is 20, so its electronic configuration is 2,8,8,2, and it has 2 electrons in the outermost shell.
Therefore to form calcium ion (Ca2+)
Calcium will lose 2 electrons and its configuration becomes 2,8,8.
Hence, the answer is the option (4).
Example 4:
Which of the following can form an Ionic bond?
1) B and Cl
2) Mg and Cl
3) Be and H
4) Si and O
Solution:
Mg has two valence electrons while Cl has seven valence electrons. Hence, one Mg atom will lose two electrons which will be gained by two Cl atoms, and the ionic compound MgCl2 will be formed.
Hence, the answer is the option (2).
Example 5:
Which one among the elements generally doesn't form an ionic compound?
1) N
2) Li
3) Na
4) Cr
Solution:
Nature of bonding in metals -
The bonding among metal atoms cannot be ionic, covalent, or van der Waals.
Li is a metal but it receives an unusual amount of attraction from their respective nucleus compared to other members of the group. This is because it has its last electron in 2s orbital which is nearest to the nucleus compared to other members. This leads to higher ionization enthalpy and lesser ionic character
Hence, the answer is the option (2).
Example 6:
The number of following factors which affect the percent covalent character of the ionic bond is_____ [JEE Main 2023]
(1) Polarising power of cation
(2) Extent of distortion of anion
(3) Polarisability of the anion
(4) Polarising power of anion
Solution:
Percent covalent character of the ionic bond
(1) Polarising power of cation
(2) Extent of distortion of anion
(3) Polarisability of the anion
Hence, the answer is the option (1).
Practice More Questions From the Link Given Below:
Summary
While explaining the characteristics of ionic compounds, some of the major physical properties related to ionic compounds that can be taken into consideration would be high melting and boiling points, electrical conductivity, and crystalline structures—physical properties underpinning their role in both natural and industrial processes. Ionic bonds play an important role in inorganic chemistry, electrochemistry, biological systems, and industrial processes like salt formation and battery technology. For competitive exams, understanding electron transfer, lattice energy, and factors like ionic size and charge is very pivotal.
NCERT Chemistry Notes:
Also Check-
Frequently Asked Questions (FAQs)
Yes. Real ionic bonds often exhibit some degree of covalent character due to polarization of the anion by the cation (Fajans’ rules help predict this).
Lattice energy is the energy released when gaseous ions form a solid ionic lattice (or conversely, energy needed to separate the lattice). Higher lattice energy generally means stronger ionic bonding.
Rarely — usually non‑metals form covalent bonds by sharing electrons. If the electronegativity difference is extremely high, partial ionic character may appear, but a full ionic bond between two nonmetals is uncommon.
Because the electrostatic forces between oppositely charged ions in the lattice are very strong, requiring large energy input to break them.
In solid form: No, because ions are fixed in lattice and can’t move.
In molten state or aqueous solution: Yes, because ions are free to move and carry charge.
An ionic bond occurs when a positively charged ion forms a bond with a negatively charged ion in which one atom gives electrons to the other. An ionic link can be seen in the chemical compound sodium chloride.
As ionic compounds are polar, they dissolve in polar solvents like water. Polar solvents breakdown ionic bonds by disrupting them. The ionic bonds can be disrupted by dissolving the ionic material in water.
Ionic bonding is a type of chemical bonding in which one atom loses valence electrons while another receives them. This exchange results in a more stable noble gas electrical state for both atoms involved. Ionic bonds are formed by the attractive electrostatic interactions between two ions with opposite charges.
According to the Duplet or Octet rule, an ionic compound is generated when atoms of metals from Groups 1 to 3 in the periodic chart lose electrons to atoms of non-metals from Groups 5 to 7 in the periodic table to complete their stable electronic configuration. During these electron exchanges, the protons of these atoms remain unchanged.
Ionic compounds have a high melting point and boil at a high temperature, whereas covalent compounds have a low melting point and boil at a low temperature.
Ionic chemicals dissolve in water, whereas covalent compounds dissolve in organic solvents such as Benzene and Phenol.
