Chemical Bonding: Definition, Types, Questions, Examples
It is a well known fact that matter is made up of atoms but under normal conditions,except noble gases, no other elements exists as an independent atom in nature. However, the smallest particles of matter which exist as independent entities are molecules. These molecules are group or cluster of atoms of same or different elements which together behave as a single unit and have characteristic properties, e.g., H2O is a molecule, composed of atoms of hydrogen and oxygen and being liquid at standard temperature and pressure is its characteristic property.
This Story also Contains
- Cause of Chemical Combination
- Lewis's symbol of elements
- Theories of Chemical Bonding
- Formation of Covalent Bonds
- Coordinate Bonds
- Types of Chemical Bonds
- Applications of Chemical Bonding
- Some Solved Examples
- Practice More Questions From the Link Given Below:
.jpg)
Chemical bonding is a very basic concept of chemistry. A description associated with the attractive forces that keep together the atomic constituents in a single unit is referred to either as a molecule or compound. It deals with complex issues like electrostatic forces, quantum mechanics, and physical properties of material. Indeed, proper knowledge of a chemical bond is indispensable to provide the full exemplification of the characteristics and behavior of matter at the atomic and molecular levels. Chemical bonding is quite an intriguing field that has seen one theory or model after another regarding explaining the nature of these forces of attraction.
Also Read -
Cause of Chemical Combination
As different atoms or ions combine to form molecules and this combination is possible due to some kind of attractive force which holds these constituent atoms together in the molecules. The attractive force which holds various constituents (atoms, ions etc) together in different chemical species is called Chemical Bond.
Since, the formation of chemical compounds takes place due to combination of atoms of various elements in differnt ways, it raises many questions such as:
- Why do some atoms combine while certain others do not?
- Why do molecules have definite shapes?
- What is the nature of force existing between the combining atoms?
To answer these questions different theories and concepts have been put forward from time to time. Some of the theories are Kossel-Lewis approach, Valence Shell Electron Pair Repulsion theory, Valence bond theory and Molecular - Orbital theory.
Atoms combine based on the following reasons:
(i) Decrease in energy: All systems in the universe tend to lose potential energy and achieve more stability. It is an observed fact that a bonded state is more stable than an unbonded state because the bonded state has lower potential energy than the unbonded state. Thus when two atoms approach each other, they combine only under the condition that there is a decrease in potential energy. When two atoms approach each other, new kinds of forces of attraction and repulsion start acting. These forces are:
- Electrons and nuclei attract each other: Attractive forces are always energetically favorable, thus an electron attracted to a nucleus is of lower energy and therefore more stable than a free electron.
- Electrons repel each other: Because of this repulsion, the energy is raised and the stability reduced.
- Nuclei repel each other: The repulsion exists between the nuclei and this also reduces the stability.
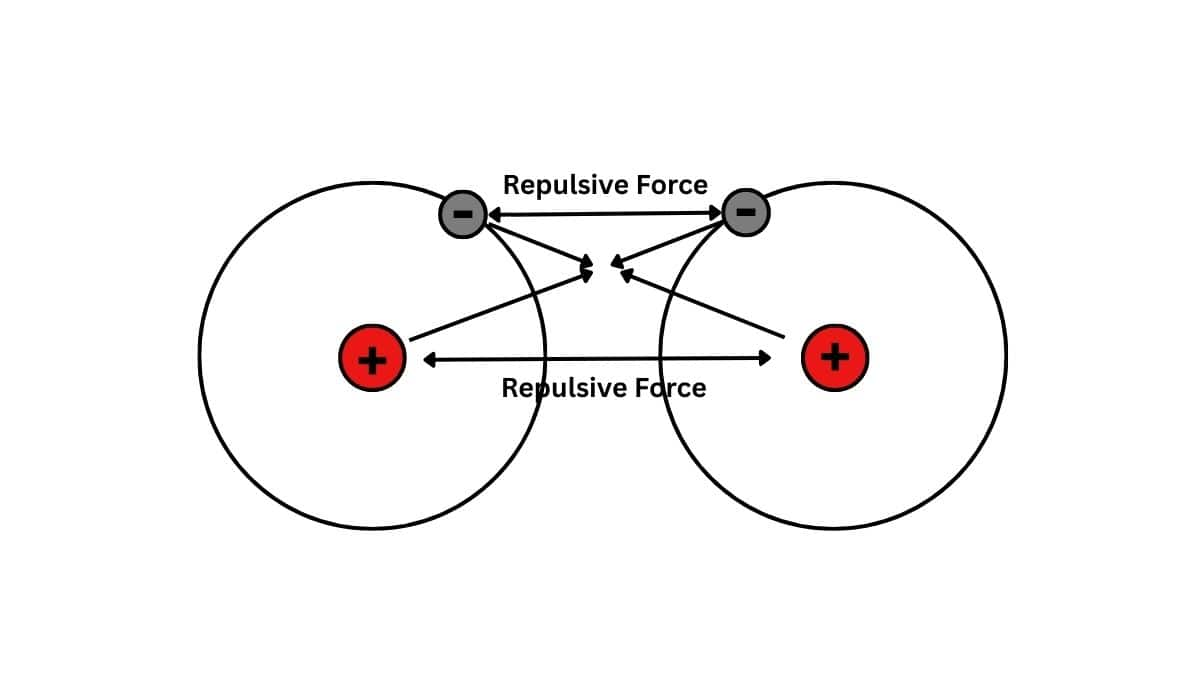
Among all these above forces, If the net result is the attraction, then the total potential energy of the system decreases and a chemical bond formation takes place. No chemical bonding is possible if the net result is repulsion.
(ii) Lewis Octet Rule: The atoms of all elements during the bond formation try to attain the stable noble gas configuration, i.e., they try to obtain either 2 electrons (when only one energy shell) or 8 electrons in their outermost energy level which is of maximum stability and hence of minimum energy. Thus, the tendency of atoms to achieve eight electrons in their outermost shell is known as the Lewis octet rule. The octet rule is the basis of the electronic theory of valency. All the noble gases like helium, neon, etc. are not active towards the bond formation because of their already filled outermost shell, in other words, their octet is already complete and thus these elements do not need to combine with other elements to complete its octet.
.jpg)
Also Check-
Lewis's symbol of elements
G.N Lewis, an American chemist introduced the simple notations to represent the valence electrons in an atom. These notations are called Lewis symbols or electron dot symbols. According to these notations the symbol of the element represents the nucleus as well as the electrons in the inner shells. The electrons in the outer shell are represented by the dots surrounding the symbol.
For explaining the formation of bonds, the Lewis symbol representation of atoms is necessary. To write the Lewis symbol for an element, we write down its symbol surrounded by several dots that are equal to the number of valence electrons. Paired and unpaired valence electrons are also indicated. The Lewis symbols for some of the elements like Chlorine, Aluminium, and Argon are mentioned below:


Significance of Lewis Symbols
The number of dots in the Lewis symbol represents the number of valence electrons. The common valencies or group valencies of the elements can be calculated from the valence electrons. The common valency of the element is either equal to the number of dots or 8 minus the number of dots. For example, the common valencies of Li, Be, B and C are 1, 2, 3 and 4 respectively while those of N, O, F and Ne are 8 minus number of dots i.e., 3, 2, 1 and 0 respectively.
Theories of Chemical Bonding
Several theories have been postulated to satisfactorily account for and describe the nature of a chemical bond formed. Each theory, however, is subject to several strengths and limitations. Some of the widely accepted theories are:
1. Lewis Theory: This theory was put forward by Gilbert N. Lewis in the year 1916. The theory underlines the valence electronic structure and the concept of sharing or transferring electrons to achieve a noble gas configuration.
There are two basic types of bonding that form the basis of the Lewis theory and it is important to understand before one can begin to draw Lewis dot structures:
1. Ionic bonding: The bond formed as a result of the electrostatic attraction between cations and anion is termed as Ionic bonding.
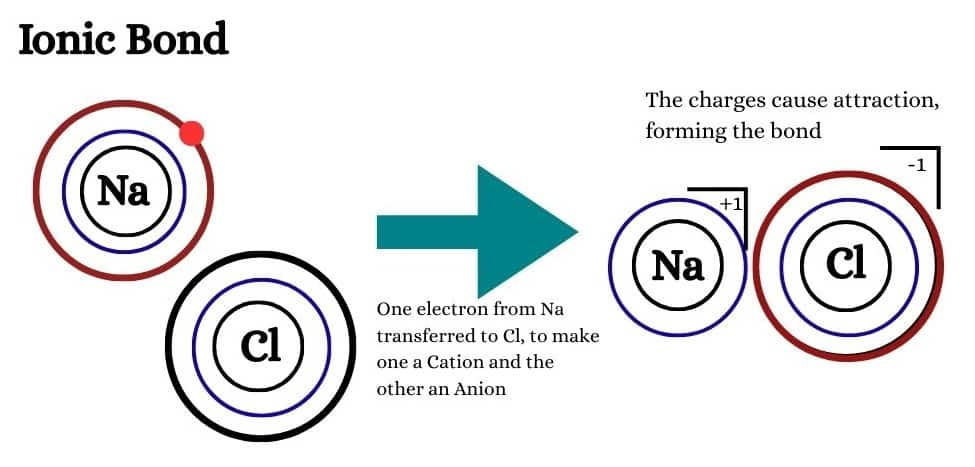
2. Covalent bonding: The gain or loss of electrons cannot take place between similar atoms. In such cases the bond is formed by mutual sharing of electrons. A force which binds atoms of same or different elements by mutual sharing of electrons is called a Covalent bond.
.jpg)
2. Valence Bond Theory: This concept was derived by Linus Pauling, who focused on the interaction of atomic orbitals and the formation of localized electron pair bonds. A few of the concepts like orbital hybridization and resonance are introduced in the theory to explain the stability as well as the directional nature of the bond.
.jpg)
3. Molecular Orbital Theory: The work of Robert Mulliken laid down in the molecular orbital theory states that delocalized molecular orbitals are brought up by the linear combination of atomic orbitals. It explains the energetics and pattern of bonds in molecules.Such theories explain the strength, directionality, and polarity of chemical bonds. The aforesaid ingredients are of immense importance in understanding the structure and properties of matter.
.jpg)
Formation of Covalent Bonds
Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. For example - the hydrogen molecule, H2, contains a covalent bond between its two hydrogen atoms. The figure given below shows the explanation of this bond. Starting on the far right, we have two separate hydrogen atoms with a particular potential energy, indicated by the red line. Along the x-axis is the distance between the two atoms. As the two atoms approach each other their valence orbitals (1s) begin to overlap. The single electrons on each hydrogen atom then interact with both atomic nuclei, occupying the space around both atoms. The strong attraction of each shared electron to both nuclei stabilizes the system, and the potential energy decreases as the bond distance decreases. If the atoms continue to approach each other, the positive charges in the two nuclei begin to repel each other, and the potential energy increases. The bond length is determined by the distance at which the lowest potential energy is achieved.
.jpg)
The potential energy of two separate hydrogen atoms (right) decreases as they approach each other, and the single electrons on each atom are shared to form a covalent bond. The bond length is the internuclear distance at which the lowest potential energy is achieved.
Coordinate Bonds
It is a special type of covalent bond in which both the shared electrons are contributed by one atom only. Such a bond is also known as a dative bond. A coordinate or a dative bond is established between two such types of atoms, out of which one has a complete octet and while the other is short of a pair of electrons. This bond is represented by “→”.
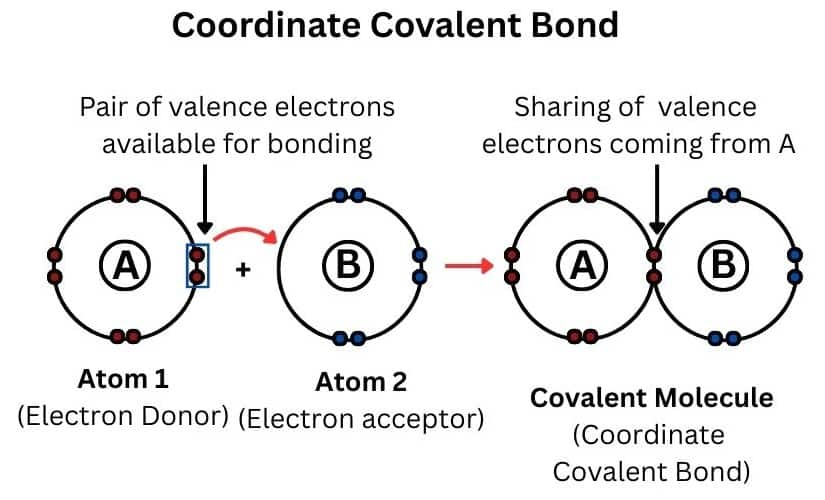
The atom that donates the electron pair is called the donor while the atom which accepts the electron pair is called the acceptor. The compounds in which the coordinate bond exists are known as complex or coordination compounds. Some example include [Pt(en)2]CO3, [Ni(H2O)6]Cl2, etc.
NCERT Chemistry Notes:
Characteristics of Coordination Compounds
The main properties of the coordination compounds are mentioned below:
- Melting and boiling points: The melting and boiling points of these compounds are higher than purely covalent compounds but lower than purely ionic compounds.
- Solubility: These compounds are sparingly soluble in polar solvents like water but readily soluble in non-polar solvents.
- Stability: The stability of these compounds is similar to the covalent compounds.
- Conductivity: Like covalent compounds, these are also bad conductors of electricity.
- Dielectric constant: The compounds containing coordinate bonds have high values of the dielectric constants.
Types of Chemical Bonds
There are many different types of chemical bonds, though they are best sorted according to what is actually occurring in the attractive forces between the atoms:
1. Ionic Bonds: Formed by a metal transferring an electron to a nonmetal, thus creating two electrically opposite ions held together by electrostatic forces.
2. Covalent Bonds: Here, both atoms combine to share electrons; this way, through electron-sharing, each of the atoms involved gets the electronic configuration of having eight electrons in its valence shell. The sharing can be equal.
3. Metallic Bonds: It is the kind of attraction that occurs between the atoms in those materials that are good conductors of electricity, such as the pure form of metals:
4. Hydrogen Bonds: Not a primary bond, hydrogen bonding is a special kind of dipole-dipole interaction that occurs when a hydrogen atom covalently bonded to an atom of high electronegativity [(nitrogen, oxygen, or fluorine)] gets close to another highly electronegative atom.
Also Read:
Applications of Chemical Bonding
Chemical bonding is a universal concept that underlines many day-to-day world applications. A comprehension of chemical bonding principles applies to the following:
1. Materials Science:, the physical properties that substances exhibit—such as electrical conductivity, malleability, tensile strength, and melting point—have their roots in the nature and strength of their chemical bonds. This is necessary knowledge to design and develop new materials with special characteristics.
2. Biochemistry: The stability and specificity of biomolecules, including proteins or nucleic acids, derive from the highly intricate patterns of chemical bonds created between the bonded atoms that form the biomolecule. This accounts for the reasons that such bonds have to be thoroughly understood so that one may progressively get to the root of the structural and functional aspects of life.
3. Nanotechnology: In such applications, concepts of chemical bonds are put to use in the manipulation and control of atoms and molecules. Having in mind the vision of desired functionalities, nanostructures, and nanodevices are sketched out, and small prototypes of the same are built, with selective formations and breaking of bonds.
4. Environmental Chemistry: Chemical bonding forms the critical underpinning to understanding and solving environmental problems. That is the formation of pollutants, contaminant behavior of soil and water, and material and process designs for sustainability.
5. Forensic Science: Chemical bond information can be used for forensic science purposes in the sense that it can identify unknown substances to determine the source of materials and serve in the courts as evidence for the presence of particular compounds on a crime scene.
Recommended topic video on (Chemical Bonding )
Some Solved Examples
Example 1:
The molecules are made of two or more atoms joined together by some force acting between them. This force is termed a:
1) Covalent bond
2) Co-ordinate bond
3) Chemical bond
4) Ionic bond
Solution: The molecules are made of two or more atoms joined together by some force acting between them. This force is termed a chemical bond. Chemical Bonds are very stable compared to other bonds.
Hence, the answer is option (3).
Example 2:
When two atoms approach each other, they combine only under condition that there should be:
1) Increase in potential energy
2) Increase in kinetic energy
3) Decrease in potential energy
4) Increase in kinetic energy
Solution: It is a fundamental truth that all-natural systems tend to lose potential energy and become more stable. It is an observed fact that a bonded state is more stable than an unbonded state. This is because the bonded state has lower potential energy than the unbonded state. Hence, when two atoms approach each other, they combine only under the condition that there is a decrease in potential energy.
Therefore, the answer is option (3).
Example 3:
Octet rule is based upon:
1) Shape of the molecules
2) Chemical inertness of noble gases
3) Energy of the molecule
4) None
Solution: As we have learned, the Octet rule is based on the chemical inertness of noble gases. Since the number of outermost electrons influences the chemical behavior of an atom, the number of eight so-called valence electrons (or two in the case of helium) means a particularly stable electron occupation. Based on the noble gases with eight valence electrons (except helium), the effort to achieve the noble gas configuration is also called the octet rule. Hence, the answer is option (2).
Example 4:
Which of the following statements is not true regarding the electronic theory of chemical bonding?
1) The theory explains the formation of a chemical bond by the sharing of electrons between two atoms
2) The theory is also known as the covalent bond theory
3) The theory assumes that atoms try to achieve stability by completing their octet or duplet
4) The theory cannot explain the formation of ionic bonds
Solution: The electronic theory of chemical bonding is based on the sharing of electrons between two atoms. It explains how the atoms in a molecule are held together by a chemical bond. This theory is also known as the covalent bond theory. According to this theory, atoms achieve stability by completing their octet or duplet configuration. However, this theory cannot explain the formation of ionic bonds, which involves the transfer of electrons from one atom to another.
Hence, the answer is option (4).
Example 5:
According to the electronic theory of chemical bonding, which of the following is not a correct statement?
1) Atoms bond together to attain a more stable electron configuration
2) A bond is formed when two atoms have overlapping orbitals
3) A bond is formed when the electronegativity difference between two atoms is less than 1.7
4) A bond is formed when the potential energy of the system is lowered
Solution: According to the electronic theory of chemical bonding, atoms bond together to attain a more stable electron configuration. A bond is formed when two atoms have overlapping orbitals and when the potential energy of the system is lowered. However, the theory does not specify an electronegativity difference of less than 1.7 as a requirement for bond formation. Therefore, option (3) is not a correct statement according to the electronic theory of chemical bonding.
Example 6:
Total number of non bonded electrons present in $\mathrm{NO}_2^{-}$ion based on Lewis theory is _________ . [JEE Main 2025]
solution:
The $\mathrm{NO}_2^{-}$(nitrite ion) has a total of 18 valence electrons, with one double bond and one single bond between nitrogen and oxygen. The double-bonded oxygen has 2 lone pairs ( 4 electrons), the single-bonded bond does oxygen has 3 lone pairs ( 6 electrons), and nitrogen has 1 lone pair ( 2 electrons). Adding them together, the total number of non-bonded electrons in $\mathrm{NO}_2^{-}$is 12.
Hence, the answer is (12).
Practice More Questions From the Link Given Below:
Conclusion
The creatures of the most insistent and fascinating area of chemistry still lie within the area of chemical bonding. The theories and types of chemical bonds explain the structure and properties of matter from the simplest molecules to the largest macroscopic structures. Their applications find wide areas that cut across materials science to biochemistry, hosting their key functions on our way of life and how they are upgrading the intellectual contributions made toward science. The development of various theories of valence and the intterpretation of the nature of chemical bonds are the main contributions to the developments in the understanding of the structure of atom, electronic configuration of elements and the periodic table. Every system tries to be stable and bonding is nature's way of lowering the energy of the syste to attain stability.
