Pi Bonds: Definition, Formula, Examples, Questions
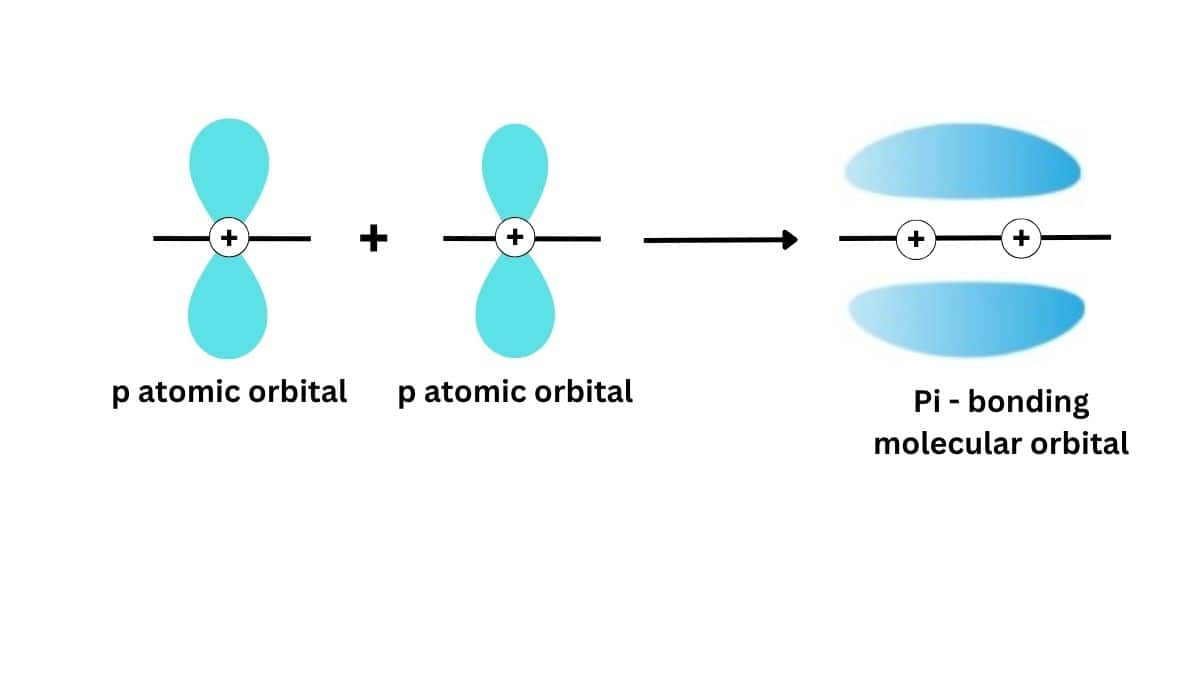
Pi bonds arise as a result of the lateral(sideways) overlap of the p orbitals, i.e., by overlapping of p-orbitals in a direction at right angles to the internuclear axis. Unsaturated hydrocarbons, having double and triple bonds, contain pi bonds. These are found in alkenes and alkynes. Such pi bonds confer unusual properties and reactivities on the compounds and hence modify their behavior in a chemical reaction. For example, the presence of pi bonds allows alkenes to undergo electrophilic addition reactions.
This Story also Contains
- Kinds of Bonding: P$\pi$-P$\pi$ and P$\pi$-D$\pi$
- p$\pi$-p$\pi$ Bonding
- $\pi$ Bonds: Real-Life Applications
- Practice More Questions From the Link Given Below:
- Summary
In this article, we will cover the concept of Pi bonds. This concept falls under the broader category of Chemical Bonding, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
Also Read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Understanding Pi Bonds
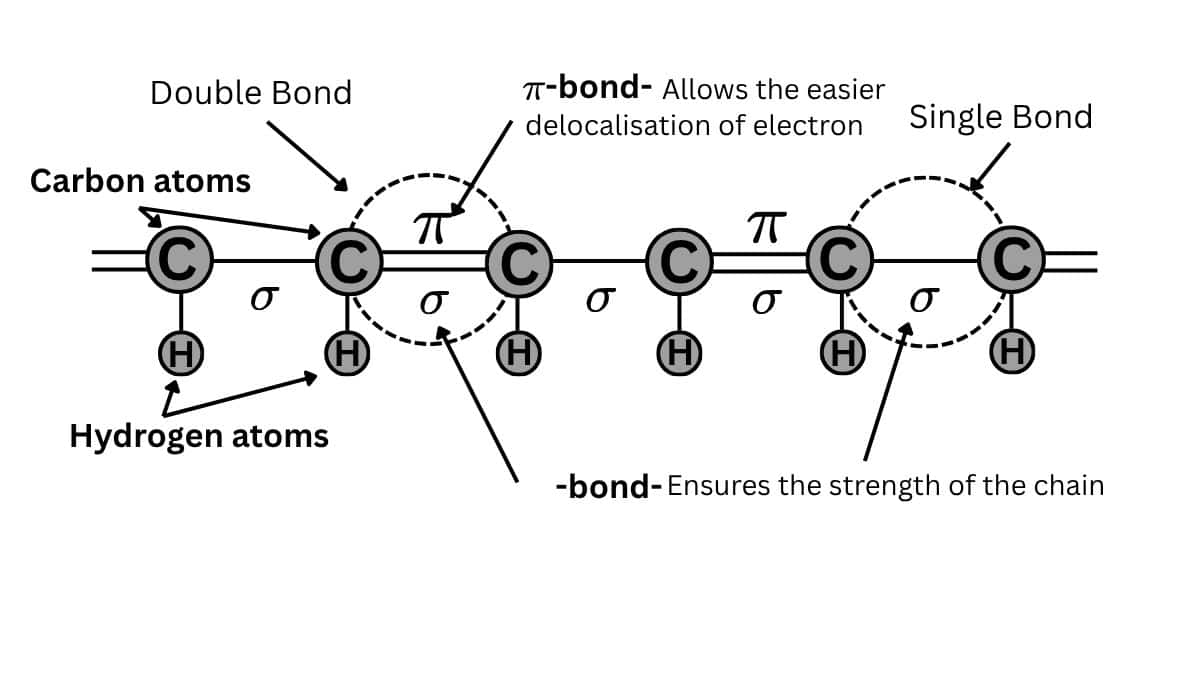
The pi bond is a covalent bond where the overlap between atomic orbitals is lateral, especially relating to p orbitals in such a way that their axis remain parallel to each other and perpendicular to the internuclear axis. This occurs when lobes of one p orbital align with those of another, resulting in a bond fundamentally different from the stronger sigma bond. Pi bonds have electron density above and below the plane of the nuclei of the bonding atoms, hence they are, in general, comparatively weaker than sigma bonds. This type of sidewise overlapping always takes place after one of the three p-orbitals has already involved in axial overlapping forming a $\sigma$ bond. The rest of two p-oritals are symmetrically placed at right angles to each other and also to the overlapped p-orbital. Therefore, these two p-orbitals can be involved in sidewise overlap.
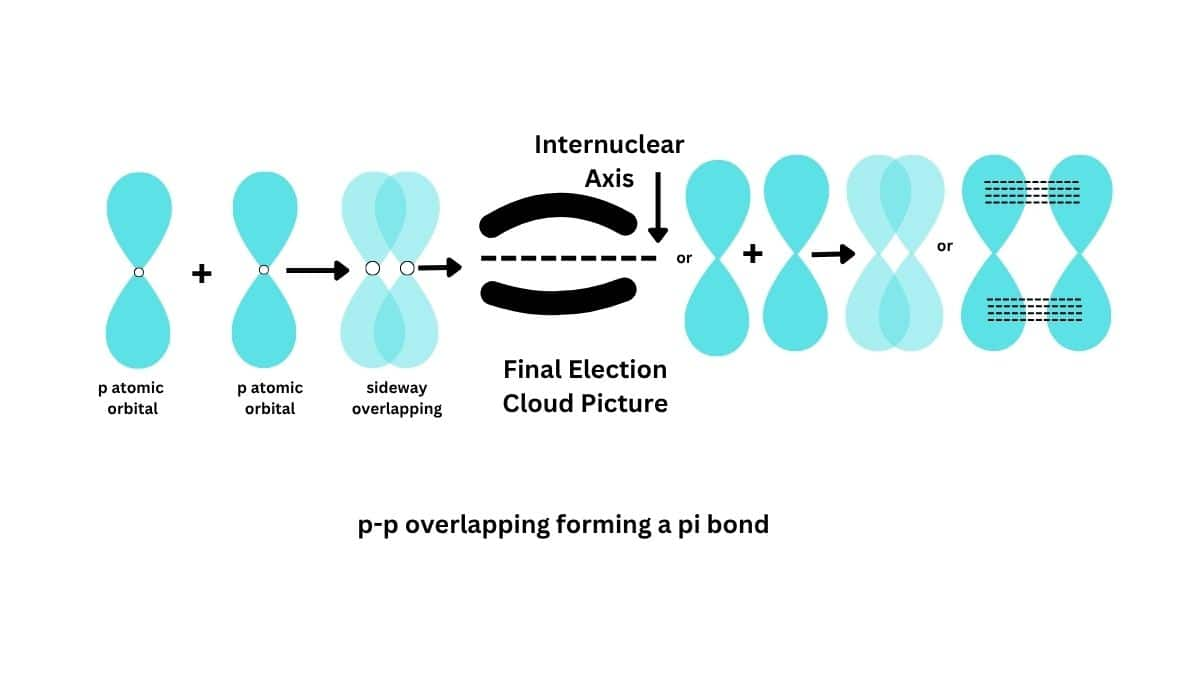 Thus, a $\pi$ bond always follows a $\sigma$ bond. A double bond, therefore, always involves a $\sigma$ and a $\pi$ bond. In the $\pi$ bond formation overlapping takes place on both sides of the internuclear axis, free rotation of atoms around a $\pi$ bond is not possible. Also, a $\pi$ bond formation shortens the bond distance between the two atoms involved. For example, $
Thus, a $\pi$ bond always follows a $\sigma$ bond. A double bond, therefore, always involves a $\sigma$ and a $\pi$ bond. In the $\pi$ bond formation overlapping takes place on both sides of the internuclear axis, free rotation of atoms around a $\pi$ bond is not possible. Also, a $\pi$ bond formation shortens the bond distance between the two atoms involved. For example, $
C-C
$ ($\sigma$ bond), $
C=C
$ (one $\sigma$ and one $\pi$ bond) and $
C \equiv C
$ (one $\sigma$ and two $\pi$ bonds) bond lengths are 154 pm, 134 pm and 120 pm, respectively.
Understanding pi bonding is important in molecular geometry because their existence restrains the rotational movement due to the fixed orientation of p orbitals on the axis of the bond. This becomes very vital in unsaturated compounds wherein the pi bonds result in specific chemical reactivity and properties.
Also Check-
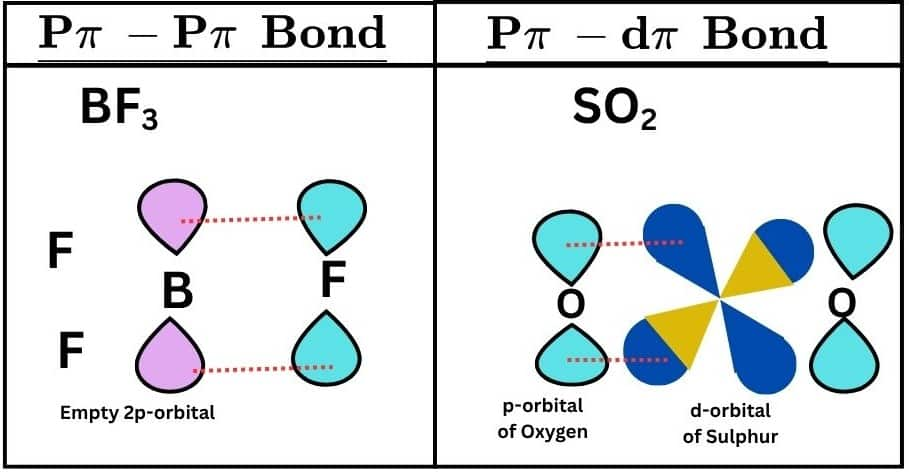
Kinds of Bonding: P$\pi$-P$\pi$ and P$\pi$-D$\pi$
The major types of $\pi$ bonding include $\pi$ (p-p) and $\pi$ (p-d) bonding.
(p$\pi$-p$\pi$) Bonding
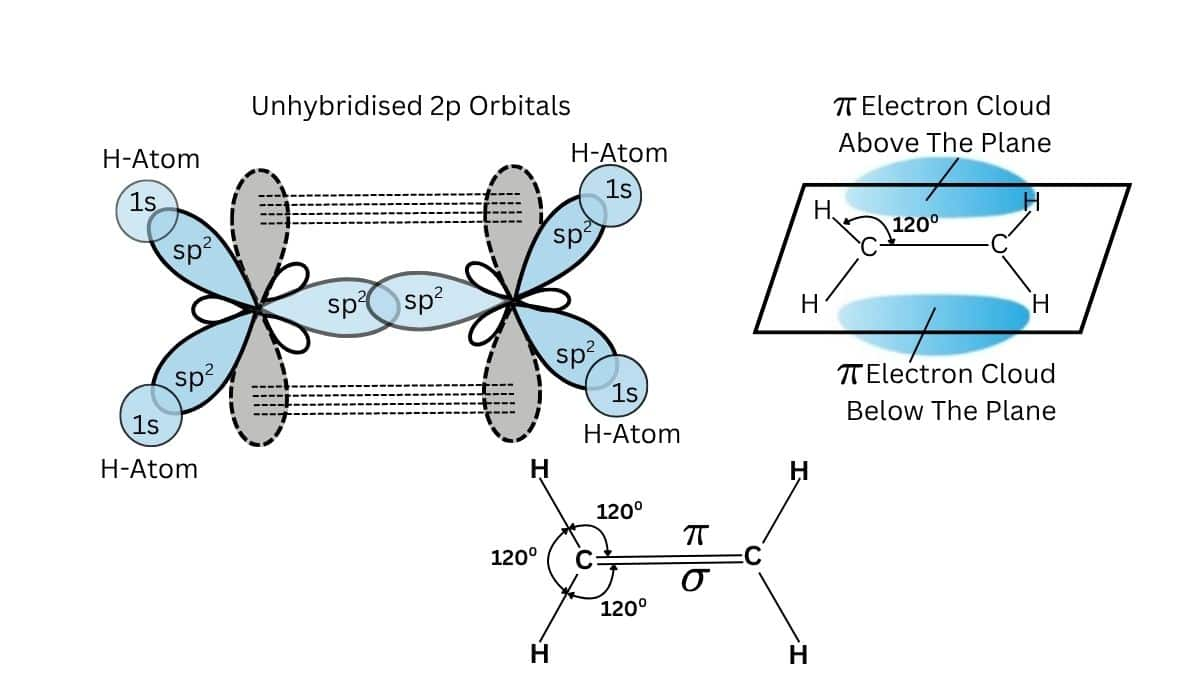
$\pi$(p-p) bonding occurs between the two 'p' orbitals of the adjacent atoms. This is so in ethylene, C₂H₄.
The carbon atoms in this molecule are sp² hybridized with one unhybridized p orbital on each carbon atom. These p orbitals overlap laterally and form the pi bond—which is above and below the plane of the molecule—to a large degree. This kind of bonding is frequent in alkenes and generally accounts for their typical reactivity, for instance, in electrophilic addition.
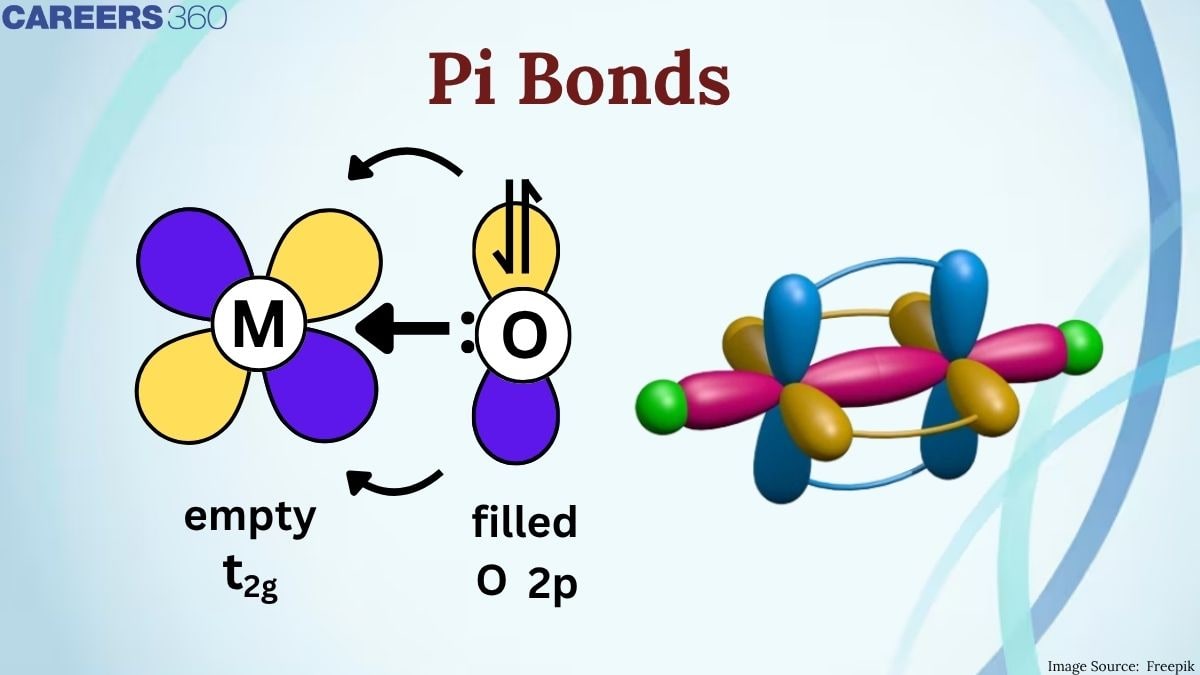
(p$\pi$-d$\pi$) Bonding
The other is $\pi$ (p-d) bonding, where p orbitals are mixed with d orbitals, which is usually observed in transition metals. This kind of bonding is quite important for the creation of metal-metal bonds in compounds like metal carbonyls and metal complexes. $\pi$ (p-d) bonds can exert a strong influence on the electronic properties of these complexes, which means their stability and reactivity.
The two kinds of $\pi$ bonding serve to illustrate just how versatile these bonds can be in different chemical settings and, therefore, their importance both in organic chemistry and inorganic.
p$\pi$-p$\pi$ Bonding
When one p-orbital of one atom contains one electron and the other atom has one electron in the p orbital, both perpendicular to the plane of the molecule, the type of bond formed is known as pπ−pπ bonding.
When one p-orbital of one atom contains one electron and the other atom has one electron, the type of bond formed is known as pπ−pπ bonding. In other words, pπ−dπ bond is formed when the p orbital of one atom and the d orbital of another atom overlap laterally.
For example, PO43- has a pπ−dπ bonding because the lateral overlap of the p-orbital of an oxygen atom with the d-orbital of a sulfur atom occurs.
NCERT Chemistry Notes:
$\pi$ Bonds: Real-Life Applications
$\pi$ bonds have several applications that stretch beyond theoretical chemistry into real life.
$\pi$ bonds are central in organic chemistry to not only the structure but also the reactivity of unsaturated hydrocarbons. Take alkenes or alkynes as examples; these are unsaturated hydrocarbons with at least one or more $\pi$ bonds. They form very essential intermediates for many synthetic reactions. The $\pi$ bond can undergo electrophilic addition that empowers the chemist to take simple molecules and then transform them into complex compounds. Hence, they become very important in the synthesis of drugs or even materials science.
Materials Science
$\pi$ bonds are important in determining the properties of various polymers. For instance, under conjugated systems where there are alternating single and double bonds, special electronic properties realize that are utilized in organic electronics and photovoltaic devices. In such materials, pi bonds allow the delocalization of electrons, hence enhancing conductivity and light absorption.
Biochemistry
The functions associated with π bonds within the structure of biomolecules are of immense importance in biochemistry. For instance, double bonds in fatty acids and the aromatic rings of nucleotides are of fundamental importance for the functions associated with the respective lipids and nucleic acids. Thus, the physical properties of such molecules result from the presence of π bonds that modulate their interactions and biological activities.
Related Topics:
Some Solved Examples
Example 1:
What is the number and type of bonds in C22- in the CaC2?
1) one $\sigma$ and 2 $\pi$
2) two $\sigma$ and one $\pi$
3) one $\sigma$ and one $\pi$
4) 2 $\sigma$ and 2 $\pi$
Solution: The structure of C22- is
$
[\ddot{c} \equiv \ddot{c}]^{2-}
$
In this ion, there is one sigma bond and two pi bonds. Therefore, correct option is 1.
Example 2:
Which one of the following does not have a pyramidal shape?
1) (SiH3)3 N
2) (CH3)3N
3) (SiH3)3
4) (CH3)3
Solution: The molecule (SiH3)3 N has a trigonal planar shape due to sp2 hybridization, while(CH3)3N does have a pyramidal shape.
Thus, the correct answer is option (1).
Example 3:
How strong is the p$\pi$-p$\pi$ bond compared to p$\pi$-d$\pi$ and d$\pi$-d$\pi$ bonds?
1) More stronger than p$\pi$ -d$\pi$ and d$\pi$-d$\pi$ bond
2) Less strong than p$\pi$-d$\pi$ and d$\pi$-d$\pi$ bond
3) More stronger than p$\pi$-p$\pi$ and d$\pi$-d$\pi$ bond
4) none of the above
Solution: The p$\pi$-p$\pi$ bond is stronger than both p$\pi$-d$\pi$ and d$\pi$-d$\pi$ bonds.
Therefore, the correct answer is option (1).
Example 4:
How many p$\pi$-p$\pi$ and p$\pi$-d$\pi$ bonds are present in SO3?
1) 2, 2
2) 2, 1
3) 1, 2
4) 1, 0
Solution: In the structure of SO3, there is 1 p$\pi$-p$\pi$ bond and 2 p$\pi$-d$\pi$ bonds.
Thus, the correct answer is option (3).
Example 5:
What is the number of p$\pi$-p$\pi$ and p$\pi$-d$\pi$ bonds respectively in P4O10?
1) 2,1
2) 1,2
3) 1, 4
4) 0, 4
Solution: In P4O10, all four π bonds (P=O) are p$\pi$-d$\pi$ bonds, as phosphorus is sp3 hybridized.
Therefore, the correct answer is option (4).
Example 6:
In the given structure, number of sp and $\mathrm{sp}^2$ hybridized carbon atoms present respectively are :
[JEE Main 2025]
1) 3 and 6
2) 3 and 5
3) 4 and 6
4) 4 and 5
Solution:
Number of sp and $\mathrm{sp}^2$ hybridised carbon atom are 3 and 5.
Hence, the correct answer is option (2).
Practice More Questions From the Link Given Below:
Summary
The paper treated $\pi$ bonds, focusing on their definitions, types, and applications in real life. $\pi$ bonds form as a result of a lateral overlap of p orbitals. Therefore, it is of prime importance in the explanation of molecular structure and reactivity. There were two notable types of bonding: p$\pi$-p$\pi$, found in alkenes and alkynes, and p$\pi$-d$\pi$, bonding seen in transition metal complexes.
Also Check-
JEE Main Previous 10 Year Questions with Detailed Solutions (2016-2025) |
Last 5 years JEE Main Chemistry question paper with solution pdf |
Frequently Asked Questions (FAQs)
Rotation around the bond would break the sideways overlap of p orbitals, which would destroy the pi bond.
Only unhybridized p orbitals can form pi bonds.
The sigma bond determines the shape and bond axis of the molecule; the pi bond only adds to electron density above and below the plane.
They lie farther from the nucleus and are more exposed, so they are easier to attack by electrophiles.
Each pi bond adds extra electron density between nuclei, increasing attraction and reducing bond length.
Draw the structure and count:
Every single bond = 1 sigma
Every double bond = 1 sigma + 1 pi
Every triple bond = 1 sigma + 2 pi
The number of unhybridized p orbitals corresponds to the number of pi bonds possible.
For example, in sp² hybridization one unhybridized p orbital remains (1 pi bond possible), and in sp hybridization two remain (2 pi bonds possible).
Single bond: 0 pi bonds
Double bond: 1 pi bond
Triple bond: 2 pi bonds
Sigma bonds form by head-on overlap; pi bonds form by side-on overlap.
Sigma bonds allow free rotation around the bond axis; pi bonds restrict rotation.
Sigma bonds are stronger; pi bonds are weaker and more reactive.
A pi bond is formed by the sideways overlap of two parallel p orbitals on adjacent atoms.
It appears in addition to a sigma bond when atoms share more than one pair of electrons (in double or triple bonds).


