Matter Particles Characteristics - Properties, Particle theory, FAQs
Our beautiful earth is the third among the total nine planets which orbits the sun. About two third of the earth surface is covered by water in the form of seas, rivers, lakes, glaciers Etc. Only one third of the surface of earth is available for human beings, animal kingdom, trees and plants to occupy. Entire earth is surrounded by earth’s atmosphere, consisting of a mixture of gases, mainly nitrogen, oxygen, carbon dioxide and other gases.
This Story also Contains
- Characteristics of matter
- Physiochemical characteristics of matter
- Physical properties of matter
- Chemical properties of matter
- Particle theory of matter
We are living and surviving on the solid earth because we are drowned in the atmosphere. Marine life exists because they are drowned in water in which oxygen is dissolved. Oxygen and water are the two essential things without which life is impossible. In general earth exists in three states. The inner central core of earth is in molten state and has a temperature of about 4000 degrees. Water on the surface of earth and below it is also in liquid state.
The atmosphere is in gaseous state. Matter is simply anything that comprises space and has mass. In general, all the matter in the universe exists in three states, solid, liquid and gas. It is intended to study matter, physicochemical characteristics, it’s fundamental theories and all about particles and much more.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
State the characteristics of particles of matter.
Characteristics of matter
Density
Color
Mass
Volume
Malleability
Melting point
Boiling point
Temperature
Physiochemical characteristics of matter
- The physicochemical properties of matter are classified as extensive and intensive properties.
- Extensive properties: any property of matter that depends on the quantity of matter that is being measured.
- For example, mass and volume.
- Intensive properties: any property of matter that can be determined by altering a matter’s molecular structure.
Physical properties of matter
Physical properties of matter are the characteristics that can be observed without altering the chemical behavior of matter. Some physical properties of matter are given below.
Density is an intensive property of matter.
Color is an intensive property of matter.
Mass is an extensive property of matter.
Volume is an extensive property of matter.
Physical constants that are melting point and boiling point are intensive properties of matter.
Chemical properties of matter
Chemical property of a matter is the ability of a matter to undergo chemical change under certain chemical behavior. Some of the chemical properties of matter are listed below.
Flammability implies whether a compound will catch fire when exposed to flame.
Heat of combustion is the energy released when a substance undergoes complete burning in presence of oxygen.
Chemical stability implies whether a substance will react in presence of air or water.
Oxidation states, the lowest energy oxidation state is the preferred state for a metal to undergo chemical changes.
Particle theory of matter
A long time ago in Greece, Democritus pointed out that matter comprises very small particles too tiny for the naked eye.
After much time gone, scientists using Democritus idea plus adding some statements created a theory called Particle theory of matter which consists of following five points
Matter is divisible in nature that is, it is made up of smaller and smaller particles.
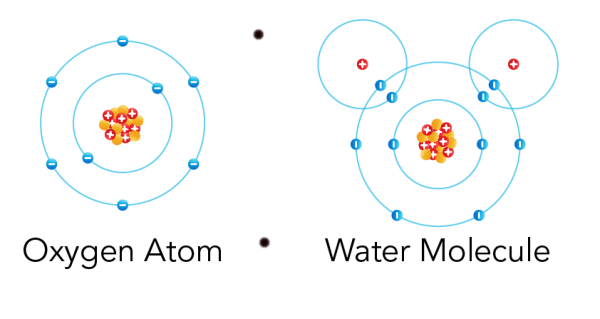
Particles of matter are continuously moving or we can say that all particles of matter are in continuous random motion. At higher temperatures the movement is quite faster because of the increase in kinetic energy with increase in temperature.
Related Topics Link, |
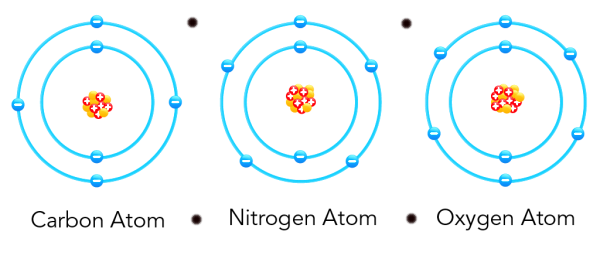
The particles of matter are attractive in nature, they attract each other.

All particles of matter comprise space in between them which is much larger in comparison to the particles itself.
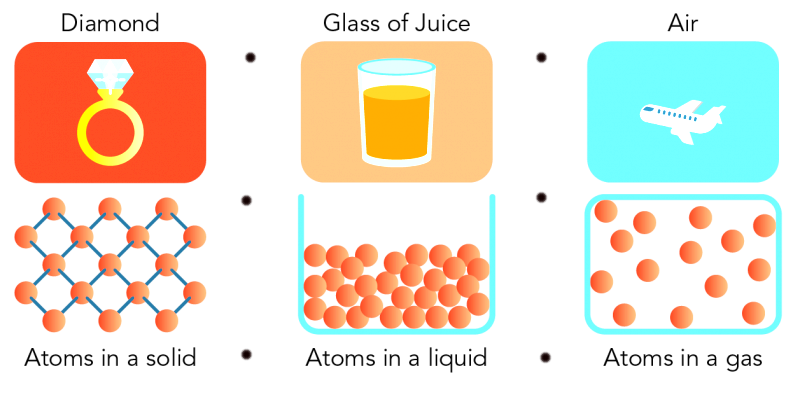
The particles of a matter are pure that is there are
identical particles in one substance.
Composite particles
Composite particle is a subatomic particle that consists of one or more elementary particles. The best examples of composite particles are the protons and neutrons which are present in the nucleus which is the central core of the atom, as they are composite, they consist of quarks. Quarks are nothing but the building blocks of matter. There are two main types of quarks: the up quark and the down quark. The proton consists of two up quark and quark and one up quark.
Coarse particle
Coarse particles are the relatively bigger particles which are found in air mainly produced by the mechanical breaking of even bigger particles. Coarse particles range in the size from 2.5 to 10 micrometer, which is the differentiating point between other particulate matter known as ultrafine particles and fine particles which range in the diameter of 0.1 to 2.5 micrometer. Some of the examples of bigger airborne particulate matter are pollen, dust, spores, and fly ash.
Evidence for particles in matter
The evidence of the presence of particles in matter comes from the diffusion experiments which consist of mixing of different substances on their own which shows the zig-zag movement of the tiny particles suspended in a gas or liquid referred to as Brownian movement or motion.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 2 Structure of Atom
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 2 Structure of Atom
- NCERT notes Class 11 Chemistry Chapter 2 Structure of Atom
Matter occupies space
What is matter? Matter is any form of object that has a suitable weight and even has a volume. Now as stated above there are three states of matter that are solid, liquid and gas, and if something is solid, it should have a definite shape and volume. The volume of a compound or an object implies the space it occupies.
The particles of matter are constantly moving
We are aware of the fact that particles are always in a state of continuous random motion, the reason behind this fact is that the particles which are in a state of motion have some kinetic energy that helps in the continuous movements or motions. Some of the best examples which illustrate the above fact are chemicals in a glass beaker, burning of paper or wood which gives off flying ashes etc.
Activity to show that particles of matter are in a continuous motion
Take two glass beakers and fill each of them with water.
Put a drop of blue ink in one glass beaker and leave it for some time undisturbed.
In the second glass beaker a teaspoon of salt and leave it undisturbed.
After sometime you will observe that the blue ink in one beaker spreads completely throughout the water present inside the beaker.
And in the second beaker too the salt completely dissolves throughout the water present inside the beaker.
This happens because the molecules are in a state of a complete random motion and when the temperature is raised to a certain limit this movement of particles happens to occur faster, which is due to the presence of kinetic energy.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
