Thomson Atomic Model: Definition, Diagram, Limitations, Example
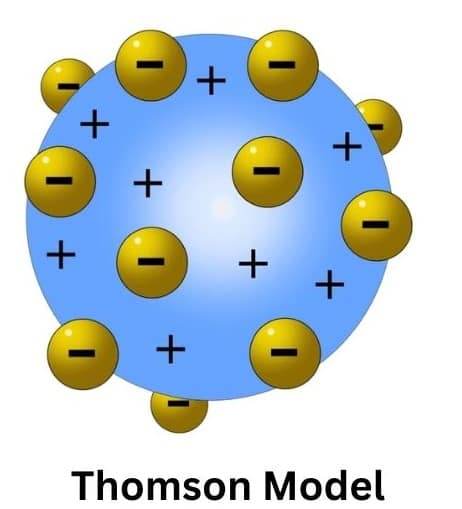
Thomson’s early‑20th‑century model of the atom was, without doubt, one of the most significant breakthroughs in the history of atomic theory. Thomson had just discovered electrons in 1897, and this was actually the first model to try talking about the internal configuration of atoms. This is commonly referred to as the "plum pudding model." Thomson proposed that an atom consists of a uniform sphere of positive charge in which the electrons are distributed more or less uniformly.
This Story also Contains
- Main Postulate of the Thomson Atomic Model
- Aspects and Examples of the Thomson Atomic Model
- Significance of the Applications of Thomson's Atomic Model
- Some Solved Examples
- Summary
Main Postulate of the Thomson Atomic Model
The main postulate in the Thomson Atomic Model is that in atoms there must exist as a positively charged sphere with the electrons being spread all over. This model originates from some hypothesis derived by Thomson from his experiments with cathode rays, which he employed to show the existence of negatively charged particles; that is electrons. The key postulates include:
1. Construction: The atom is a sphere of positive charge in which the charge is uniformly spread. There are negative electrons spread throughout the positive sphere, more or less as in "plum pudding" or a watermelon.
2.Charge Neutrality: The total negative charge of the electrons is equal in magnitude to the net positive charge so that the atom is electrically neutral.
3. Mass Distribution: The mass of an atom, according to Thomson, is distributed uniformly throughout the sphere—completely a different thing from earlier models of the atom, none of which considered the structure of atoms at all.
Although the most eventual model that arose came from Thomson, it was heavily flawed when compared with its theory regarding the stability of the atom and arrangement of the subatomic particles. The two failures:
- It's inability to explain the stability of the atom.
- The inability of the model to account for results of experimental evidence like that obtained in the experiment carried out by Rutherford in which he scattered alpha particles on gold foil, were important reasons why the model declined with time as more accurate models were sought.
Aspects and Examples of the Thomson Atomic Model
The Thomson Atomic Model brought up so many points that were to assist in the formulation of the subsequent atomic theories. Among the best points about this model is the visualization of an atom as a homogeneous entity, so radically opposite to the approach of the models immediately preceding it: those that tend to treat atoms as indivisible units.
It might be more helpful to conceptualize this model by considering a few analogies.
So in this, there are two analogies:
- The positive charge was represented as the pudding, and the electrons represent plums which were scattered all around.
- The proper analogy of a watermelon. The flesh of the watermelon in red represents the positive charge, and the seeds represent the electrons.
The analogies provided a descriptive, albeit nebulous, toehold for those uninitiated in atomic theory to start considering the model. Beyond how utterly flawed it was, the Thomson model was simplistic. It did absolutely nothing to further the analysis of Rutherford's findings: that at the heart of every atom existed a small, dense core known as the nucleus and that within the infinitely spacious void that surrounded this core, negatively charged electrons orbited around countless volumes of space.
This model was proposed by J.J Thomson in 1898. According to this model, the atom possesses a spherical shape in which a positive charge is uniformly distributed and the electrons are embedded into it in such a manner that it gives the most stable electrostatic arrangement. This model is also known by various names like plum pudding, raisin pudding or watermelon. One important feature of this model is that it could explain the overall neutrality of the atom but it was not consistent with the results of experiments carried out later by different scientists.
Significance of the Applications of Thomson's Atomic Model
The model of Thomson is too simple in nature, but because of this, it has some drawbacks as it was essential for the realization in which atomic number is structured and used for different purposes.
1. Foundation for Future Models: The model laid open a foundation for future theories, mainly distinguishing Rutherford and Bohr models, which evolved ideas put down by Thomson. A proper understanding and transition of these models is essential for students and scholars, as it seems a bit vaguely described in the literature.
2. Educational Importance: The first introduction model that is followed in the education systems, starting from school students studying chemistry and physics at school up to college and further classes in the university, is basically to understand the elementary structure of an atom that tends to proceed towards several intense theories related to it.
3. Technological Consequences: The knowledge concerning electrons has led to various technologies that are now considered to be parts of the present-day life of any electrical device, for example, every semiconductor. In fact, the principles devised from Thomson's atomic model are still being practically utilized up to the present in certain fields of study on quantum mechanics and materials science.
4. Historical Context: It's really the bread and butter of the history of science, showing how scientific investigation grows via experiment and then further refinement of theory. It shows the role that skepticism plays and how further advance of science is the product of no less than further empirical tests.
Basically, Thomson's Atomic Model is one very important step in the development of the atomic theory. Although it was replaced much later, its more developed concept on an electron and the idea of charge neutrality developed research like no other.
Also Read,
Recommended Topic Video on (Thomson Atomic Model)
Some Solved Examples
Example 1
Question: Thomson assumed the atom to be a spherical body in which:
1) Electrons are evenly distributed in the sphere.
2) Electrons are unevenly distributed in a sphere.
3) Both.
4) None.
Solution:
Thomson's atomic model suggests that the atom possesses a spherical shape with a positive charge uniformly distributed throughout. The electrons are embedded within this sphere, leading to a stable electrostatic arrangement. Therefore, the correct answer is option (2): Electrons are unevenly distributed in a sphere.
Example 2
Question: Plum pudding model was proposed by:
1) E. Rutherford
2) N. Bohr
3) J J Thomson
4) Debroglie
Solution:
The plum pudding model was proposed by J.J. Thomson in 1898. In this model, the atom is depicted as a sphere with a uniformly distributed positive charge, with electrons embedded within it. This model, also known as the raisin pudding or watermelon model, explains the overall neutrality of the atom. Hence, the answer is option (3): J J Thomson.
Example 3
Question: Thomson’s model of the atom is inspired by which fruit:
1) Mango
2) Apple
3) Watermelon
4) Papaya
Solution:
Thomson's atomic model is often compared to a watermelon, where the positively charged sphere represents the fruit's flesh, and the electrons represent the seeds embedded within it. Therefore, the correct answer is an option (3): Watermelon.
Example 4
Question: Electric field strength that balances gravitational force on an electron is:
1) $5.7^* 10^{-10} \mathrm{v} / \mathrm{m}$
2) $5.7^* 10^{-11} \mathrm{v} / \mathrm{m}$
3)$4.7^* 10^{-10} \mathrm{v} / \mathrm{m}$
4) $4.7^* 10^{-11} \mathrm{v} / \mathrm{m}$
Solution
As we learn
Millikan's oil drop method: Millikan measured the charge on an electron by this oil drop method.
- wherein
We adjust the voltage in the plates so that the electrical attraction upward just balances the force of gravity downward.
At equilibrium
eE = mg
$E=\frac{m g}{e}=\frac{9.1 * 10^{-31} * 10}{1.6 * 10^{-19}}=5.7 * 10^{-11} v / m$
$10^{-19}=5.7 * 10^{-11} v / m$
Hence, the answer is the option (2).
Example 5
Question: The electric field used in Thomson's experiment had a strength of $8.0 \times 10^3 \mathrm{~N} / \mathrm{C}$. If a cathode ray particle with a mass of $9.11 \times 10^{-31} \mathrm{~kg}$ and a charge of $-1.6 \times 10^{-19} \mathrm{C}$ was deflected by an angle of 30 degrees in the electric field, what was the speed of the particle before entering the field?
1) $1.4 \times 10^7 \mathrm{~m} / \mathrm{s}$
2) $2.0 \times 10^7 \mathrm{~m} / \mathrm{s}$
3) $2.5 \times 10^7 \mathrm{~m} / \mathrm{s}$
4) $3.0 \times 10^7 \mathrm{~m} / \mathrm{s}$
Solution:
The electric force experienced by the cathode ray particle in the electric field can be given as $\mathrm{F}=\mathrm{qE}$ where q is the charge of the particle and E is the electric field strength. The force on the particle causes it to undergo circular motion, and the radius of the circular path can be given as $\mathrm{r}=\mathrm{mv}^2 / \mathrm{Bq}$ where m is the mass of the particle, v is its velocity, and hence, the answer is the option (write option no.). is the magnetic field strength. In this case, there is no magnetic field, so we can use the equation for the radius of the circular path in the electric field, which is $r=m v^2 / 2 q E \sin (\theta)$, where$
\theta
$ is the angle of deflection. Equating this to the radius of the circular path gives:
$\frac{m v^2}{2 q E \sin \theta}=\frac{m v^2}{B q}$
Simplifying and solving for v, we plug in the values given in the problem, and we get
$\begin{aligned} & \mathrm{v}=\sqrt{\frac{2\left(-1.6 \times 10^{19} \mathrm{C}\right)\left(8.0 \times 10^3 \mathrm{~N} / \mathrm{C}\right)}{\left(9.11 \times 10^{31} \mathrm{~kg}\right) \sin \left(30^{\circ}\right)}} \\ & \mathrm{v} \approx 2.5 \times 10^7 \mathrm{~m} / \mathrm{s}\end{aligned}$
Hence, the answer is the option (3).
Practice More Questions From the Link Given Below:
Summary
The atomic model which was developed in 1900 by J.J. Thomson, the notion of a positive sphere of embedded electrons, negatively charged, added up to the former notion of an atom, which was further improved and developed into an actual physical model upon which further theories about the atom succeeded. It did have many apparent failures, though: the increase in atom stability and the varying number of possible arrangements of subatomic particles. However, it did keep an essential groundwork to let scientists and theories develop further on the atom. Models like the plum pudding and watermelon models could enable the visualization of atomic structure and were hence open for learning.
