Atomic Number Mass Number - Definition, Example, Formula & Calculation, FAQs
Have you ever wondered why each element in the periodic table has a unique identity? What exactly does the atomic number of an element tell us about its structure, and how is it related to the number of protons in the nucleus? You will get these answers by reading this article on Atomic number and Atomic mass. Atomic number of an element can be defined as the number of protons present in the nucleus which can be denoted by the symbol Z, and simply as we can better understand the atomic number by taking an example i.e. if we see the hydrogen atom which contains one proton so the atomic number of hydrogen atom will be one, similarly for the sodium atom it will be 11 owing to the total 11 proton in its nucleus.
This Story also Contains
- Atomic Number
- Atomic Mass
- Atomic Number and Atomic Mass Of Elements
- Calculation of Atomic Number and Mass Number
- Summary
- Some Solved Examples
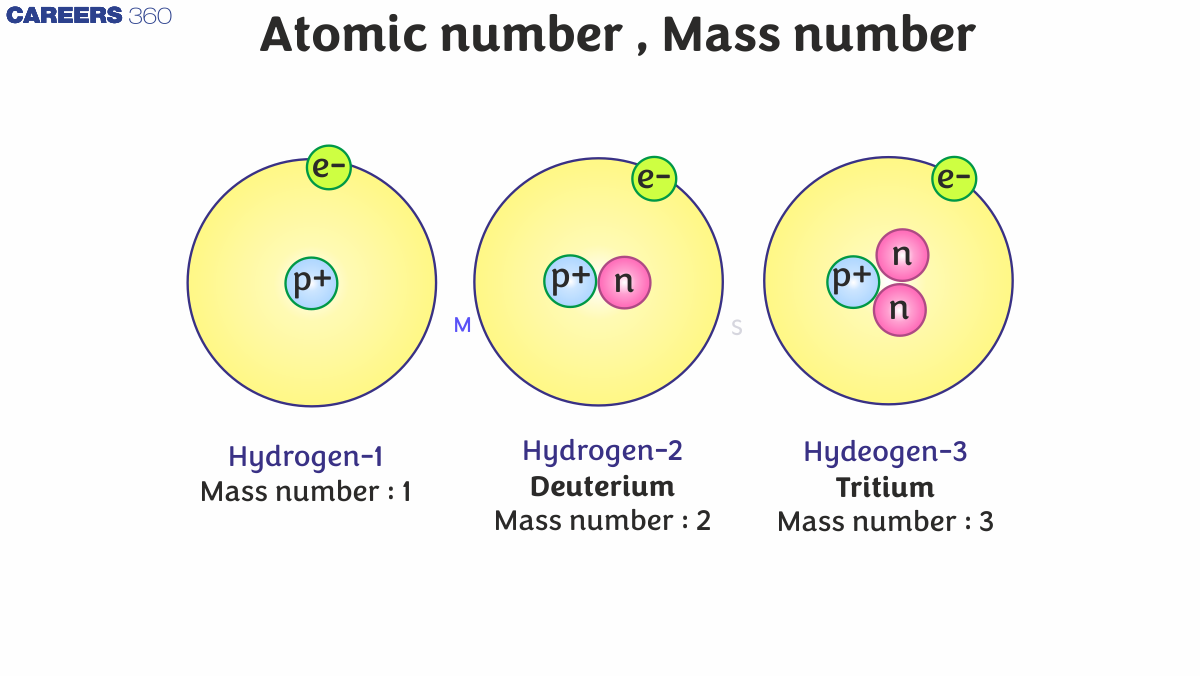
Atomic Number
Atomic Number is the number of protons present in the nucleus of an atom. It is denoted by Z. Since an atom is electrically neutral, the atomic number also equals the number of electrons in the atom.
The atomic number uniquely identifies an element. For example:
-
Hydrogen has Z = 1 (1 proton).
-
Oxygen has Z = 8 (8 protons).
-
The arrangement of elements in the modern periodic table is based on their atomic number.
So, from the above fact we can say that the dependency of the atomic number is based only on the protons in the element, not on the electrons or neutrons, so by using this statement, we can create a relation mathematically as;
Z = no. of protons = no. of electrons
Below is the complete representation of an element with its symbol, atomic number, and mass number.
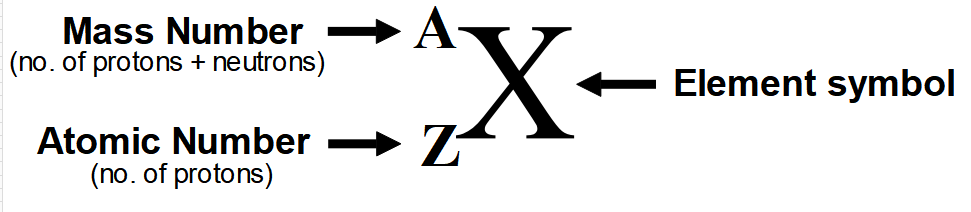
Atomic Mass
Atomic mass of an element is the sum of protons and neutrons in the nucleus and is denoted by the symbol "A" as shown in the above image, so mathematically we can write as:
A = Protons + Neutrons
This combination of protons and neutrons is also called nucleons.
We can also write.
A = Z + Neutrons
Neutrons = A – Z
In the periodic table, the hydrogen element is the only element that does not have a neutron and has only one proton.
|
Related Topics |
Atomic Number and Atomic Mass Of Elements
The atomic mass of an element is actually very small because atoms are extremely small. Today, we have sophisticated techniques, such as mass spectrometry, for determining atomic masses fairly and accurately. But in the 19th century, scientists could determine the mass of an atom relative to another atom experimentally, as mentioned earlier.
|
Name of Elements |
Symbol |
Atomic number |
Number of Electrons |
Number of Protons |
Number of neutrons |
Atomic Mass |
|
Hydrogen |
H |
1 |
1 |
1 |
- |
1 |
|
Helium |
He |
2 |
2 |
2 |
2 |
4 |
|
Lithium |
Li |
3 |
3 |
3 |
4 |
7 |
|
Beryllium |
Be |
4 |
4 |
4 |
5 |
9 |
|
Boron |
B |
5 |
5 |
5 |
6 |
11 |
|
Carbon |
C |
6 |
6 |
6 |
6 |
12 |
|
Nitrogen |
N |
7 |
7 |
7 |
7 |
14 |
|
Oxygen |
O |
8 |
8 |
8 |
8 |
16 |
|
Fluorine |
F |
9 |
9 |
9 |
10 |
19 |
|
Neon |
Ne |
10 |
10 |
10 |
10 |
20 |
|
Sodium |
Na |
11 |
11 |
11 |
12 |
23 |
|
Magnesium |
Mg |
12 |
12 |
12 |
12 |
24 |
|
Aluminium |
Al |
13 |
13 |
13 |
14 |
27 |
|
Silicon |
Si |
14 |
14 |
14 |
14 |
28 |
|
Phosphorus |
P |
15 |
15 |
15 |
16 |
31 |
|
Sulphur |
S |
16 |
16 |
16 |
16 |
32 |
|
Chlorine |
Cl |
17 |
17 |
17 |
18 |
35.5 |
|
Argon |
Ar |
18 |
18 |
18 |
22 |
40 |
|
Potassium |
K |
19 |
19 |
19 |
20 |
39 |
|
Calcium |
Ca |
20 |
20 |
20 |
20 |
40 |
The above table contains the element name, atomic number, atomic mass, number of protons, number of electrons, and number of neutrons, so we can easily differentiate between them, in inorganic chemistry we can also find such type of data and we can clearly learn the increasing or decreasing number of atom or mass by going from up to down the periodic table. An element’s number is adequate to the number of protons in the nuclei of any of its atoms. The table of elements in the periodic table gives the atomic number of every element. The number may be an integer, usually written above the chemical symbol of every element within the table. The number for hydrogen is 1 because every hydrogen atom has a proton.
The number for helium is because every helium atom has 2 protons, the number of electrons within the table, which are arranged according to the increasing number of protons within the nucleus. Accordingly, the protons, which are usually adequate for the number of electrons within the neutral atom, are additionally the number. For a while, let’s say iron has 26 electrons, it means it has its atomic number 26. As we have discussed above the number of an element is the sum of protons and neutrons. Now, we will discuss the calculation of the atomic number and mass no.
Also check-
Calculation of Atomic Number and Mass Number
Our great scientists calculated the mass of an atom by means of the mass number from the isotopes of the elements that occur naturally. Often, the decimal number is obtained in the results. For instance, the atomic mass of chlorine is 35.45amu due to its various isotopes.
Atomic Number :
$Z=\text { Number of protons }$
In a neutral atom,
$\text { Number of protons }=\text { Number of electrons }$
Example:
- Hydrogen $\rightarrow 1$ proton $\rightarrow \mathbf{Z}=\mathbf{1}$
- Oxygen $\rightarrow 8$ protons $\rightarrow \mathbf{Z}=8$
Mass Number:
$A=\text { Number of protons }+ \text { Number of neutrons }$
or
$A=Z+\text { Number of neutrons }$
Example:
For Oxygen-16:
- Number of protons $=8$
- Number of neutrons $=8$
$A=8+8=16$
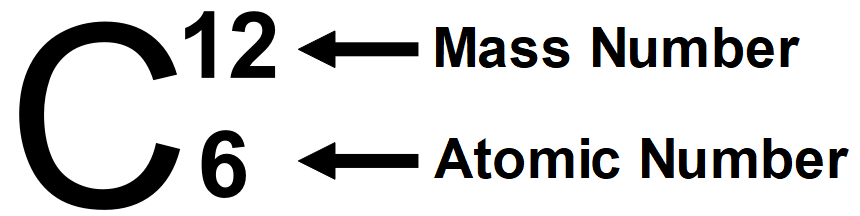
Summary
It is well known that protons are present within the nucleus of an atom. The atomic number of any element is determined by the number of protons present in an atom; it's denoted by ‘Z’. All atoms of a component have an equivalent number, Z. In fact, elements are defined by the number of protons they possess. For hydrogen, Z = 1, because in an atom, just one proton is present within the nucleus. Similarly, for carbon, Z = 6. Therefore, the number is defined as the total number of protons present within the nucleus of an atom.
By studying the properties of the subatomic particles of an atom, we can easily say that the total mass of an atom is primarily thanks to protons and neutrons alone. That's by protons and neutrons are also called as nucleons. Therefore, the total mass of an atom resides in its own nucleus. For instance, the mass number of carbon is 12amu due to its 6 protons and 6 neutrons. In nature, a variety of atoms of some elements are identified, which have the same number of atoms but different mass numbers, which are called isotopes. For example hydrogen element has its isotopes named deuterium and tritium, denoted by simply D and T.
Let us consider two elements, calcium (Ca), atomic number 20, and argon (Ar), atomic number 18. The number of electrons in these atoms is different, but the nucleon number of both these elements is 40.
Also read -
| NCERT Solutions for Class 11 Chemistry |
| NCERT Solutions for Class 12 Chemistry |
| NCERT Solutions for All Subjects |
Some Solved Examples
Solution: The answer is the $\mathrm{NO}^{+}$and (2) $\mathrm{N}_2$
Explanation: Both species have the same number of electrons but a different number of protons. Hence, they are isoelectronic with CO .
Hence, the answer is option (1).
Solution:
Atomic number (z) = number of protons in the nucleus of an atom
Mass number (A) = sum of neutrons and protons in the nucleus
Isotopes = Atoms having the same atomic number but a different mass number.
Isobars = Atoms with the same mass number but different atomic number
The answer is option (3)
1:3, because we know that chlorine is made up of two isotopes that have atomic masses of 35 u and 37 u in the ratio 1:3.
Hence, the answer is option (3).
1) i and ii
2) ii and iii
3) (correct) iii and iv
4) i and iv
Solution:
As we learnt in
Isotopes -
Atoms having the same atomic number but different mass numbers.
i) atomic number =6, mass numbers different
ii) atomic number =17, mass numbers different
iii) atomic number different, mass numbers different
iv) atomic number different, mass numbers different
Therefore, clearly iii & iv do not isotopes
Hence, the answer is option (3).
Question 4: Which of the following atoms/ions contains 23 Nucleons (protons and neutrons)
1) $M g$
2) (correct) Na
3) Ca
4) V
Solution:
As we learned
$11^{N a^{23}} A=23$
So the sum of protons and neutrons $=23$
Hence, the answer is option (2).
Question 5: CO is isoelectronic with
1) (correct) $\mathrm{NO}^{+}$
2) $N_2$
3) $\mathrm{SnCl}_2$
4) $\mathrm{NO}_2{ }^{-}$
Solution:
The answer is the $\mathrm{NO}^{+}$and (2) $\mathrm{N}_2$
Explanation: Both species have the same number of electrons but a different number of protons. Hence, they are isoelectronic with CO.
Hence, the answer is option (1).
Practice More Questions Withthe Link Given Below
Frequently Asked Questions (FAQs)
| ATOMIC NUMBER | ELEMENT | ATOMIC MASS |
|---|---|---|
| 1 | Hydrogen | 1.008 |
| 2 | Helium | 4.0026 |
| 3 | Lithium | 6.94 |
| 4 | Beryllium | 9.0122 |
The atomic number can be defined as the protons present in the nucleus which can be denoted by the symbol Z.
Atomic mass of an element is the sum of protons and neutron in the nucleus and is denoted by the symbol A and mathematically it can be written as
A = Protons + Neutrons
Hydrogen is the element having atomic number =1.