Molischs Test - Definition, Test Procedure & Reaction, Uses, FAQs
What is Molisch’s test?
Molisch test was named after an Austrian botanist Hans Molisch. Molisch test for carbohydrates is chemical test sensitive to carbohydrates. The carbohydrates can be bound to lipids or proteins or can be in Free State. It requires precision for the detection of carbohydrates.
Molisch meaning
Molisch in English means biochemical test for the presence of sugar.
State the principle of molisch test
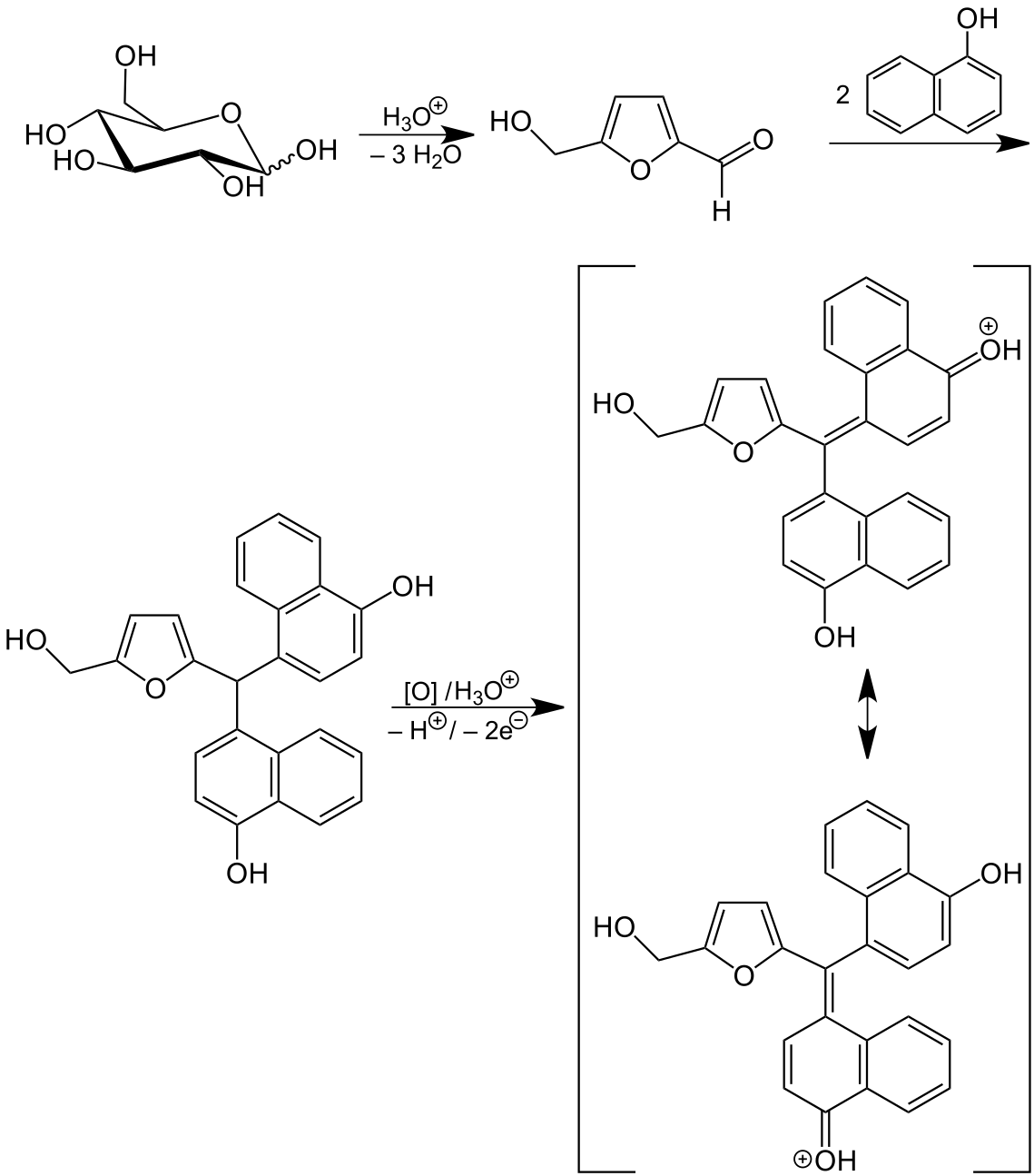
Molisch test is based on the principle that concentrated acid helps catalyzing the dehydration of sugars (carbohydrates) to produce furfural (from pentose) or hydroxymethylfurfural (from hexoses) where furfural is an aldehyde with -CHO group attached to the 2-position of furan ring. This furfural molecule condenses with two molecules of phenol (although generally α-naphthol is used but occasionally other phenols like resorcinol and thymol can also be used) to form purple or violet colored complex at the interface of acid-liquid layer.
All carbohydrates such as monosaccharides, disaccharides, and polysaccharides (besides triose and tetrose) show positive reactions. Both polysaccharides and disaccharides are first converted into monosaccharides by strong concentrated acid. Other compounds like glycoproteins (or glycolipids) and nucleic acid can give positive reactions as they are first hydrolyzed into monosaccharide by the action of strong acids. Sometimes a green ring can be observed due to presence of impurities in the reagent which might react with the phenol or the acid present in solution. If a solution of concentrated sugar is used, then a rind ring is observed due to charring of sugar by the acid.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Molisch reagent preparation
Molisch’s reagent is a solution mixture of phenol dissolved in ethanol. This mixture should always be prepared fresh. The exact Molisch reagent composition can be listed as 3.75g of α-naphthol dissolved in 25 ml solution of 99% ethanol.
Molisch test procedure
Molisch test is performed by combining a small amount of Molisch reagent (i.e., α-naphthol dissolved in solution of 99% ethanol) with the solution that needs to be examined. A small amount of concentrated sulfuric acid is poured along the sides of the test tube after combining Molisch reagent and ethanol. The mixture should not be stirred to form two separate layers of acid and test solution along the junction of the test tube.
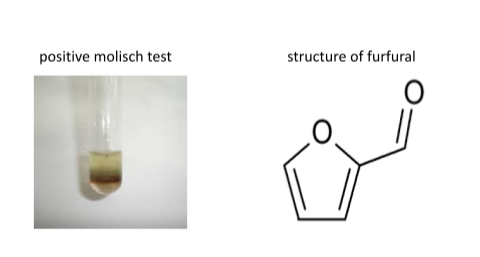
A purple or violet ring observed at the interface of two liquid layers indicating the presence of carbohydrates in the given solution. When the contents of the test tube (test tube with violet ring at junction) are shaken in a stream of cold water, a deep purple solution is obtained which on dilution with cold water yields a violet precipitate. On shaking the contents of the test tube again and adding a small amount of suspension to an excess of concentrated ammonia, a dull brown color is obtained.
Molisch test procedure with accurate measurements can be performed as:
Take a test solution in a test tube along with 2ml distilled water.
Separate the solution in 4 test tubes to ensure backup if the reaction doesn’t go according to procedure.
In each of the test tubes, add 2 drops of Molisch reagent.
Add 1ml of concentrated sulfuric acid slowly along the sides of each test tube and leave the solution mixture for 2 minutes. DO NOT STIR THE SOLUTION. If the concentrated acid is not poured slowly with precautions, charring of carbohydrates takes place and a black ring may form.
(Tip: to ensure stability of test tube incline the test tube in a test tube holder to restrict movement and prevent charring)
A reddish violet ring is observed between the interface of the acid layer and the solution layer.
Also Read:
Molisch test reaction
The reaction can be summarized as:
Polysaccharides H2SO4→ Monosaccharides (pentoses + hexoses) conc. H2SO4→ furfural (and hydroxyfurfural) ∝-naphthol→ Triarylmethane compounds (violet color)Polysaccharides are first converted into monosaccharide in the presence of strong acid. These monosaccharides are converted into furfural; an aldehyde which on reaction with 2 molecules of phenol produces a violet colored complex.
Furfural test vs. Molisch test
Furfural test; also known as rapid furfural test, is based on the same principle that concentrated acid helps catalyze the dehydration of sugars (carbohydrates) to produce furfural (from pentose) or hydroxymethylfurfural (from hexoses). Although in furfural test or rapid furfural test concentrated hydrochloric acid is used in the place of concentrated sulfuric acid followed by boiling of solution.
The same procedure as the Molisch test is applied. Initially to the dilute solution of sugar, ethanolic naphthol is added followed by slow addition of concentrated hydrochloric acid. Finally, the solution is boiled. In case of fructose or sucrose, a violet color is immediately obtained when the solution starts boiling but in case of glucose the appearance of color is delayed and may not be produced before one minute boiling.
Uses of Molisch test
The Molisch test is used to determine the presence of carbohydrates in different samples.
Molisch test can also be used to distinguish between byproducts of reaction which form carbohydrates as byproducts.
Limitation of Molisch test
Sugars like trioses and tetroses do not contain five carbon atoms which are required for formation of furfural which is a five membered ring compound. And thus does not give a positive Molisch test result.
Some other organic substances which can form furfurals like oxalic acid, citric acid, lactic acid, formic acid etc., can also give a positive Molisch test result which means the Molisch test is not specific for carbohydrates.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: