Acetic Acid - Overview, Structure, Properties & Uses, FAQs
Acetic acid is a carboxylic acid group organic compound having the molecular formula CH3COOH. In this compound, a group of methyl is attached to a functional carboxyl group. The main element of vinegar is acetic acid. Mixer of Acetic acid and water forms the vinegar solution, of which 5% to 20% in volume is acetic acid. The acetic acid has a pungent odor and a sour taste. In this article, we will study the structure of acetic acid, its preparation, physical and chemical properties, along with some solved examples from past years.
This Story also Contains
- Structure of Acetic acid
- Ethanoic Acid Structure
- Preparation of Acetic Acid
- Physical Properties of Acetic Acid
- Chemical Properties of Acetic Acid
- Uses of Acetic Acid
- Some Solved Examples
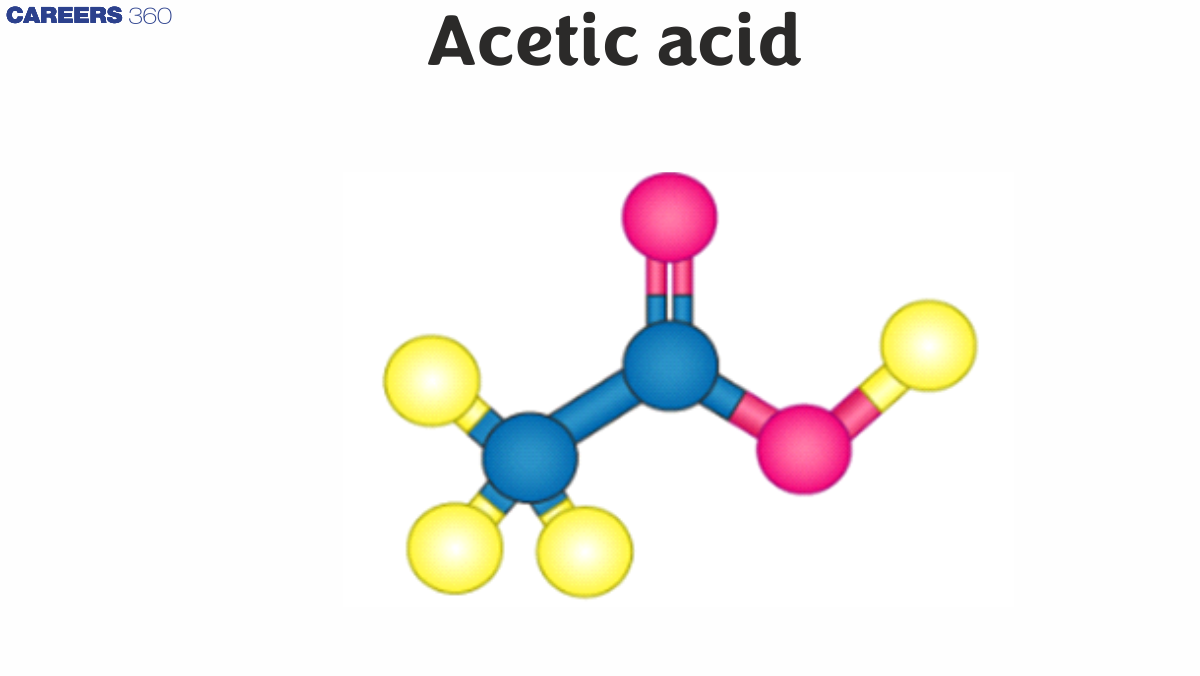
Acetic acid is found in or produced and excreted by acetic acid bacteria, especially Acetobacter and Clostridium acetobutylicum. These bacteria are ubiquitous in food, water, and soil. Acetic acid is naturally produced when fruits and other foods deteriorate. The IUPAC name of acetic acid is Ethanoic acid.
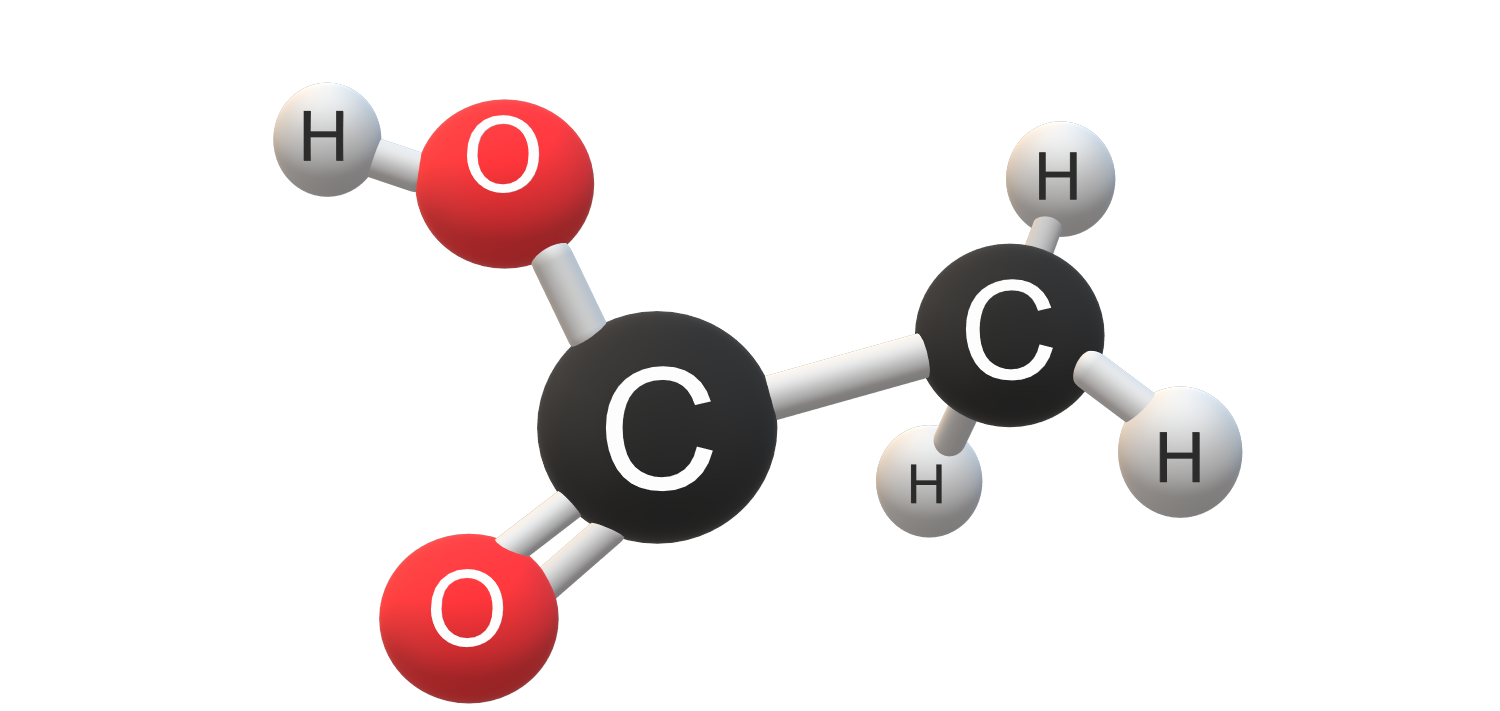
Structure of Acetic acid
Let’s see the Acetic acid structure. Ethanoic acid is the simplest carboxylic acid.
-
The acetic acid structure is defined as CH3COOH.
-
In the solid state, acetic acid is a molecular chain in which individual molecules are connected by hydrogen bonds.
-
The temperature of its dimer in the vapor state is approximately 120 °C.
In the liquid phase, its dimer exists in a dilute solution.
|
Related Topics |
Ethanoic Acid Structure
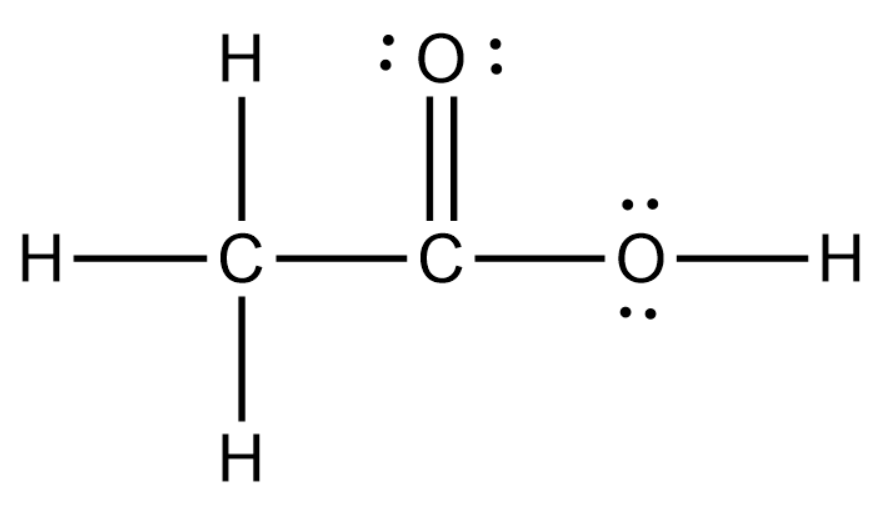
Lewis structure of CH3COOH (Acetic acid)
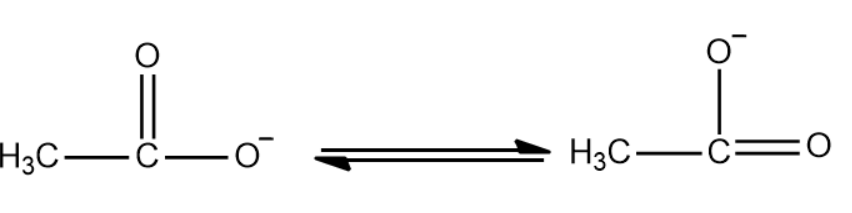
Resonance structure of CH3COOH (Acetic acid)
CH3COOH CH3COO-+H+
Preparation of Acetic Acid
The methanol carbonation produces acetic acid industrially. The chemical equations are provided below for the three steps involved in this process.
-
CH3OH + HI → CH3I+ H2O
-
CH3I + CO → CH3COI
-
CH3COI + H2O → CH3COOH + HI
Here, the reaction between methanol and hydrogen iodide produces an intermediate methyl iodide. This intermediary reacts with carbon monoxide and is treated with water to provide the product with acetic acid. It is important to note that for step 2 of this process, a carbonyl metal complex should be used as a catalyst.
Physical Properties of Acetic Acid
-
Acetic acid has a pungent vinegar taste and a bitter taste.
-
Colorless liquid.
-
It boils at 391 K.
-
It has a liquid density of 1049 g/cm³.
-
It can be mixed with water, alcohol, and ether in any ratio.
-
It is soluble in water, emits heat, and reduces its volume.
-
It is corrosive and can cause blisters in contact with the skin.
Chemical Properties of Acetic Acid
-
The carboxyl functional group in acetic acid causes the compound to ionize according to the following reaction: CH₃COOH ⇌ CH₃COO⁻ + H⁺
-
The acidity of acetic acid is based on an equilibrium reaction that releases the aforementioned protons.
-
In an aqueous solution, the acid dissociation constant, pKa, of acetic acid is 4.76.
-
CH3COO−, acetate is the acetic acid conjugate base.
-
Acetic acid will not dissociate completely, because it can be seen that the pH of an acetic acid solution with a concentration of 1.0 M is 2.4.
-
Acetic acid is a polar protic solvent with a dielectric constant of 6.2 in liquid form.
Uses of Acetic Acid
Ethanoic acid in humans' everyday lives is a very important organic compound. The following is a list of important uses of acetic acid.
-
Because of its antibacterial qualities, acetate acid is used as an antiseptic.
-
Ethanoic acid is involved in the production of radiation fiber.
-
Acetic acid, by direct injection into the tumour, has been used to treat cancer.
-
As the main component of vinegar, many vegetables are used for pickling.
-
Ethanoic acid is used for the manufacture of rubber. It is also used in the production of different perfumes.
-
It is used in large numbers in VAM production (vinyl acetate monomer).
Also check-
Some Solved Examples
Question 1. Which of the following gives the correct increasing order of acidic strength?
(a) Water <Acetic acid <Hydrochloric acid
(b) Water <Hydrochloric acid <Acetic acid
(c) Acetic acid <Water <Hydrochloric acid
(d) Hydrochloric acid <Water <Acetic acid
Solution:
Hydrochloric acid is a mineral acid and ionizes completely in water, which is why it is a strong acid.
Acetic acid is an organic acid and ionizes only partially in water; hence, it is a weak acid. Water is neutral.
Thus, the order of acidity is
water < acetic acid < hydrochloric acid.
Hence, the correct answer is option 1
Question 2. Acetic acid can be obtained by oxidation of which of the following?
A. Methane
B. Acetaldehyde
C. Ethanol
D. Both B and C
Solution:
Ethanol oxidizes (primary alcohol → aldehyde → acid)
$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH} \rightarrow \mathrm{CH}_3 \mathrm{CHO} \rightarrow \mathrm{CH}_3 \mathrm{COOH}$ (with strong oxidant).
Acetaldehyde oxidizes to acetic acid:
$\mathrm{CH}_3 \mathrm{CHO}+[\mathrm{O}] \rightarrow \mathrm{CH}_3 \mathrm{COOH}$.
Methane (CH4) is not oxidized to acetic acid under ordinary lab/industrial conditions. So both B and C give acetic acid.
Hence, the correct answer is option (D)
Question 3. Which reagent gives acetic acid on the oxidation of ethanol?
A) PCC
B) $\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7 / \mathrm{H}_2 \mathrm{SO}_4$
C) $\mathrm{H}_2 / \mathrm{Pd}$
D) $\mathrm{PCl}_5$
Solution:
PCC (pyridinium chlorochromate) oxidizes primary alcohol → aldehyde, not further to acid (mild oxidant).
$\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7 / \mathrm{H}_2 \mathrm{SO}_4$ (acidified dichromate) is a strong oxidizing agent that oxidizes ethanol fully to acetic acid:
$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}+2[\mathrm{O}] \rightarrow \mathrm{CH}_3 \mathrm{COOH}+\mathrm{H}_2 \mathrm{O}$.
H₂/Pd reduces, PCl₅ converts alcohols to chlorides.
Hence, the correct answer is option (B)
Frequently Asked Questions (FAQs)
Acetic acid, also known as ethanoic acid has chemical formula CH3COOH.
The common name of CH₃COOH is acetic acid.
Acetic acid uses are across different fields:
1. Food Industry: Vinegar Production and Food Preservation
2. Chemical Industry: Chemical Reagent
3. Medical Uses: Antiseptic and antibiotics.
4. Household uses: Cleaning Agent
Acetic acid is generally safe when used in diluted forms, such as in vinegar (about 4-8% concentration). It is commonly used in food, cleaning, and other household applications without causing harm. However, safety depends on its concentration as at higher concentration, it can be an eye, skin, and mucous membrane irritant.
