Photochemical Reaction - Laws, Principles, Examples Applications and FAQs
Have you ever wondered why some reactions occur only in the presence of sunlight? The answer lies in this article, which contains the important sections of the chapter on photochemistry. Here we will discuss the laws of photochemistry with its different types and examples. Application of photochemistry, such as photosensitisation, and the difference between photochemical and thermal reactions. The branch of chemistry that deals with the absorption of light energy is known as photochemistry.
This Story also Contains
- Photochemistry
- Photochemical Reaction
- Principles Of Photochemical Reaction
- Laws Of Photochemistry
- Photosensitization
- Examples Of Photochemical Reaction
- Exploring The Applications Of Photochemical Reactions
- Comparing Photochemical And Thermal Reactions
- Photochemical And Electrochemical Reactions: A Comparative Study
- Some Solved Examples
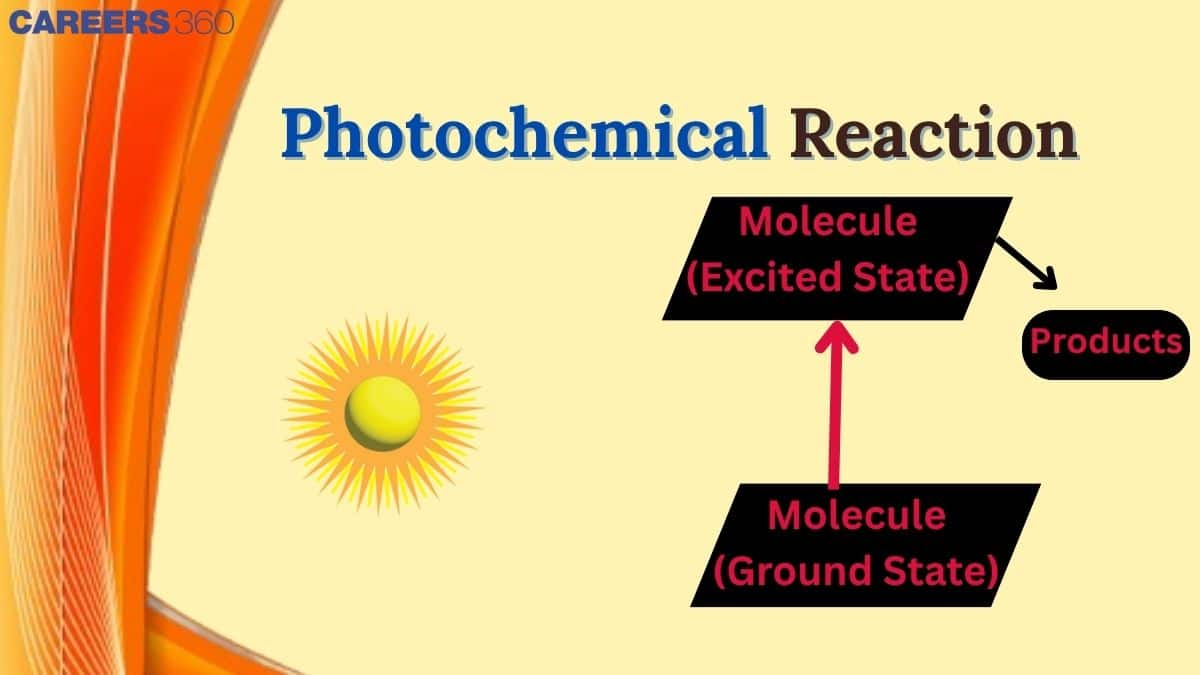
Molecules retain energy and jump into an excited state when they absorb light, usually UV or visible light. Regaining energy leads to a more excited state, and reactions occur that may not occur otherwise. We will discuss Photochemical reaction, laws, principles, examples, applications, and comparison of Photochemical and Thermal reactions that reveal the remarkable role of light in chemistry in this article, and some solved examples are also given. To know more, scroll down.
Photochemistry
Photochemistry is a branch of chemistry that deals with the chemical reactions that are triggered by the absorption of light radiation, primarily in the ultraviolet (UV) or visible spectrum. These reactions differ from thermal reactions as they rely on photons rather than heat to carry out molecular changes. Light absorption causes molecules to jump into their higher-energy excited electronic states, which causes different reaction pathways that cannot be achieved by traditional thermal activation. The properties of the molecules when they are in this state are completely different from the previous state
Photochemical Reaction
Developments in the field of photochemistry took place in the 1800s. In the year 1817, German physicist Theodor von Grotthuss put forward theoretical ideas about the photochemical process.
Importance Of Photochemical Reaction
Photochemical reactions are of great importance to support life on Earth. Chemical changes in the atmospheric gases are done by solar radiation and modified by the particles present. The photochemical reactions of the upper atmosphere help us to study the depletion of the ozone layer, acid rain, and global warming.
Photochemical reactions have many advantages when compared to other types of reactions. Photochemical reactions need sunlight and it is present abundantly. Complex organic molecules such as proteins and nucleic acid are synthesized by the simple gaseous molecules like methane, ammonia, and carbon dioxide after undergoing photochemical reaction.
Principles Of Photochemical Reaction
The principles of photochemical reaction are based on photochemistry. When a molecule absorbs photons, it gets excited to a higher energy state. This process is known as photoexcitation. The photochemical reaction depends on two laws:
-
Grotthuss-Draper Law: This law states that all light radiations do not produce chemical reactions. Some increase the kinetic energy of molecules while some are reemitted.
-
Stark-Einstein Law: This law states that each molecule of absorbing substance absorbs one photon or quantum of the radiation in the primary process.
-
Beer-Lambert’s Law: This law gives a linear relationship between absorbance and concentration of the species. According to this law, if a monochromatic light is passed through a solution of an absorbing substance, then the rate of decrease in intensity of radiation is directly proportional to the thickness of the tube and concentration of the solution.
Laws Of Photochemistry
Quantum yield
The efficiency of a particular reaction taking place by photochemical process is given by the term called quantum yield. Quantum yield is defined as “the ratio of the number of molecules reacting in a given time to the number of quanta absorbed in the same time.” Many photochemical reactions are complex and occur differently, so the quantum yield is usually specific for each reaction.
Different Types Of Photochemical Reactions
The different types of photochemical reactions are as follows:
-
Photo-dissociation reaction:
$\mathrm{X}_2+h \nu \rightarrow 2 \mathrm{Y}^{\bullet}$
- Isomerization reaction:
$\mathrm{X}+h \nu \rightarrow \mathrm{Y}$
- Photo-addition reaction:
$\mathrm{X}+\mathrm{Y}+h \nu \rightarrow \mathrm{XY}$
- Photo-substitution reaction:
$\mathrm{X}-\mathrm{Y}+\mathrm{Z}+h \nu \rightarrow \mathrm{X}-\mathrm{Z}+\mathrm{Y}$
- Photo-redox reaction:
$\mathrm{X}+\mathrm{Y}+h \nu \rightarrow \mathrm{X}^{+}+\mathrm{Y}^{-}$
Photosensitization
Photosensitization involves two processes in which a donor species absorbs light to form an excited species followed by energy transfer to a suitable acceptor species. The excited species, thus excited indirectly, can undergo various processes known as photosensitized reactions or photosensitization. There are two types of photosensitized reaction: One is electron transfer and second is energy transfer reaction.
Examples Of Photochemical Reaction
Photochemical reaction examples are:
-
Photosynthesis: During this process, the chlorophyll pigment in plants takes up the energy (hν) from the sun. Solar energy along with water convert carbon dioxide to glucose and oxygen. Artificial light is also used to carry out this process.
$6 \mathrm{CO}_2+6 \mathrm{H}_2 \mathrm{O}+\mathrm{hv} \rightarrow \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+6 \mathrm{O}_2$
-
An example of a photochemical decomposition reaction is seen in photography. When light falls on silver chloride (AgCl) or silver bromide (AgBr) it produces an image. During this reaction Silver halides (AgX) decomposes into silver (Ag) and halogen (X2).
$\begin{aligned} & 2 \mathrm{AgCl}+\mathrm{hv} \rightarrow 2 \mathrm{Ag}+\mathrm{Cl}_2 \\ & 2 \mathrm{AgBr}+\mathrm{hv} \rightarrow 2 \mathrm{Ag}+\mathrm{Br}_2\end{aligned}$
-
Solar cells release the energy in the form of electricity by using the light energy from the sun.
-
Formation of vitamin D when skin is exposed to sunlight.
Photochemical Reaction in the Atmosphere
In the atmosphere, there are some gaseous substances that change the chemical composition of air. From the kinetic molecular theory of gases, it is observed that the molecules present in the atmosphere move and collide with each other continuously. During the daytime, the atmosphere receives continuous solar radiation. As a result, these molecules absorb the light and photochemical reactions take place. These reactions have an important role in studying the nature of chemical species in the atmosphere. The oxidation reactions in the atmosphere are due to the reaction with solar energy.
Related Topic Links
Exploring The Applications Of Photochemical Reactions
Some applications of photochemical reactions are:
-
Photochemical reactions are used for the synthesis of vitamins, drugs, and fragrances.
-
It is used for free-radical chlorination, nitration etc.
-
It is used for the formation of an anti-malaria drug
-
It is used for the preparation of benzyl chloride
-
It is used for the synthesis of various synthetic organic compounds
-
It is used for the development of optical bleaches.
Comparing Photochemical And Thermal Reactions
|
Photochemical Reaction |
Thermal Reaction |
|
Takes place by the absorption of radiation (photons) by molecules |
Takes place by the absorption of heat energy, generally by increasing the temperature of the reaction medium |
|
A light source is used |
The heat source is used |
|
An adequate light source is required |
Reaction can occur even in the absence of light |
|
Temperature causes no effect |
Temperature causes a direct effect |
|
Catalyst is not required to accelerate the reaction rate. But, a high intensity of light can increase the rate of reaction. |
Most reactions require a catalyst to increase the rate of the reaction |
Photochemical And Electrochemical Reactions: A Comparative Study
|
Photochemical Reaction |
Electrochemical Reaction |
|
Takes place by the absorption of radiations (photons) by molecules |
Takes place by the passage of electric current |
|
Light source is used |
Electricity is the source used |
|
Photosynthesis is an example |
Reactions in an electrical cell is an example |
Also Read:
Some Solved Examples
Question.1 Which of the following laws governs the quantitative relationship between light absorbed and the chemical reaction occurring?
a) Boyle’s Law
b) Grotthuss-Draper Law
c) Hess’s Law
d) Henry’s Law
Solution:
The Grotthuss-Draper Law states that only the light absorbed by a substance can bring about a photochemical change. Unabsorbed light has no effect on the reaction.
Hence, the correct answer is option b) Grotthuss-Draper Law
Question.2 Which principle explains that one photon of light activates only one molecule in a photochemical reaction?
a) Kirchhoff’s Law
b) Beer-Lambert Law
c) Stark-Einstein Law
d) Le Chatelier’s Principle
Solution:
The Stark-Einstein Law of Photochemical Equivalence states that each absorbed photon activates one molecule, initiating the reaction (quantum yield may vary later).
Hence, the correct answer is option c) Stark-Einstein Law
Question 2: Which of the following is not an example of a photochemical reaction?
a) Photosynthesis in plants
b) Photography (formation of silver image)
c) Rusting of iron
d) Chlorine and hydrogen reaction in sunlight
Solution:
Rusting of iron is a slow electrochemical process, not a photochemical one. Photochemical reactions specifically require light energy to proceed.
Hence, the correct answer is option c) Rusting of iron
Practice More Questions from the link given below:
Also check-
Frequently Asked Questions (FAQs)
An example of a photochemical reaction involving water is the photolysis of water during photosynthesis.
$2 \mathrm{H}_2 \mathrm{O} \xrightarrow{\text { light, chlorophyll }} 4 \mathrm{H}^{+}+4 e^{-}+\mathrm{O}_2$
The example of a photochemical reaction is the reaction between hydrogen and chlorine in the presence of sunlight
$\mathrm{H}_2+\mathrm{Cl}_2 \xrightarrow{\text { sunlight }} 2 \mathrm{HCl}$
Photochemical reactions require light absorption and often proceed via excited electronic states.
Thermal reactions rely on heat energy and follow the ground-state potential energy surface.
Φ = 1: One photon leads to one reaction (follows the Stark-Einstein law).
Φ > 1: Chain reaction (e.g., radical reactions).
Φ < 1: Energy loss via fluorescence, phosphorescence, or non-radiative decay.
Fluorescence: Fast emission (ns timescale), same spin state (singlet→singlet).
Phosphorescence: Slow emission (ms-s), involves spin change (triplet→singlet).