Rutherford Atomic Model And Its Limitations
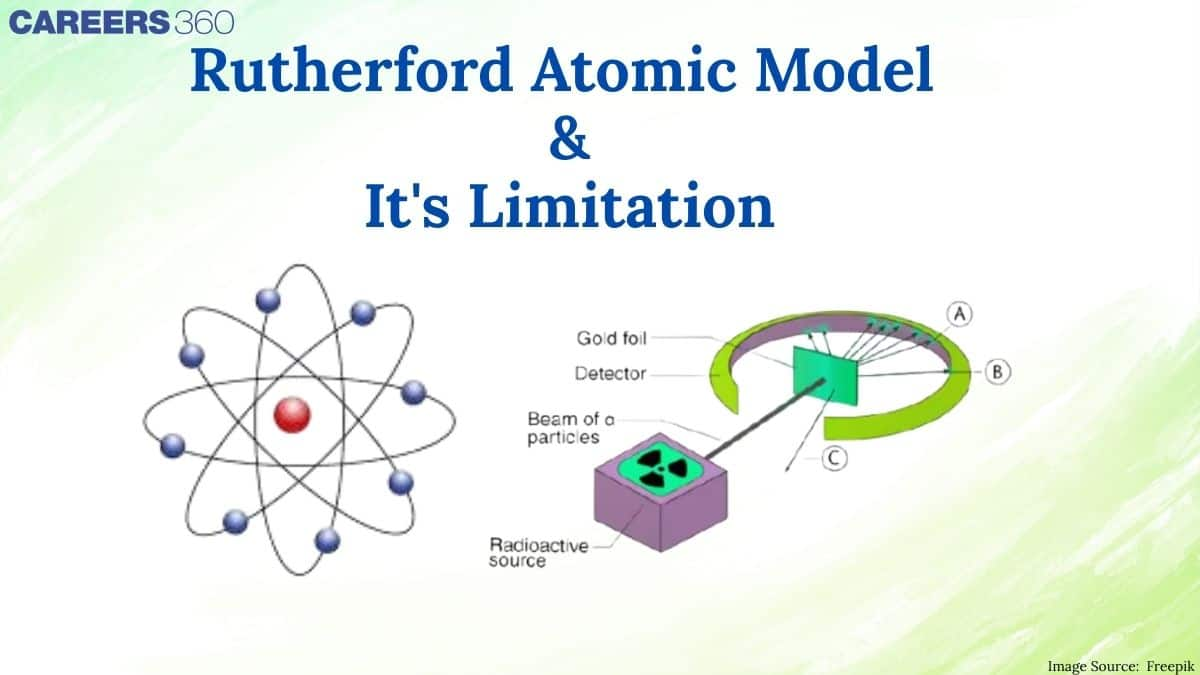
The famous Rutherford's Alpha Scattering Experiment, which clarifies the fundamentals of atomic structure, was conducted by Ernest Rutherford. Using a radioactive source, Rutherford directed high-energy streams of α-particles towards a 100 nm-thick gold sheet. According to Rutherford, alpha scattering experiment was the most incredible event that ever happened in his life. It was almost as incredible as if you fire a fifteen onch shell at a piece of tissue paper and it came back to hit you. To find out how the alpha particles were deflecting Rutherford allowed a narrow beam of alpha particles to fall on a very thin gold foil. This gold foil had circular florescent zinc sulphide screen around it.
This Story also Contains
- Rutherford's Alpha Adventure: Unveiling What's Inside the Atom?
- Observations of the Rutherford's Experiment:
- Rutherford's Atomic Model's Conclusion:
- Limitations of the Rutherford's Model:
- Solved Examples Based On Rutherford's α Scattering Experiment
- Practice More Questions From the Link Given Below:
- Conclusion
In this article, we will cover the concept of Rutherford's Alpha particle Experiment, observations including the conclusion based on the experiment. This concept falls under the broader category of Atomic structure, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE and more.
Rutherford's Alpha Adventure: Unveiling What's Inside the Atom?
A stream of high-energy α–particles from a radioactive source was directed at a thin foils (thickness ∼ 100 nm) of metals like gold, silver, platinum or copper. Slits were used to get a fine beam. The presence of alpha particle at any point around the thin foil of gold after striking it was detected with the help of a circular zinc sulphide screen. The point at which an alpha particle strikes this screen, a flash of light was given out.

Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Observations of the Rutherford's Experiment:
- Most of the α–particles (99.9%) passed through the gold foil without undergoing any deflection indicating that most of the atom is space.
- A small fraction of the α–particles was deflected by small angles indicating that the positive charge is concentrated in a minimal volume.
- Very few α–particles (∼1 in 20,000) bounced back, that is, were deflected by nearly 180° thus confirming the size of the nucleus to be very small.
Rutherford's Atomic Model's Conclusion:
-
Most of the part of the atom is empty and the atom is spherical.
-
Each atom consists of a small, heavy, positively charged portion located at the centre, known as the nucleus.
-
All positive charge of atoms (i.e. protons) are present in the nucleus and electrons move around the nucleus in circular orbits.
-
Electrons and the nucleus are held together by electrostatic forces of attraction.
Limitations of the Rutherford's Model:
-
According to Maxwell's theory, a moving charged particle under acceleration radiates energy and thus the electron must spiral into the nucleus, but that does not occur. The stability of the atom was not explained by Rutherford's model.
-
Rutherford's model does not provide any information about the position of the electron or its energy.
Also Read:
Recommended Topic Video on (Rutherford Atomic Model and its Limitations)
Solved Examples Based On Rutherford's α Scattering Experiment
Example 1: If the Thomson model of the atom was correct, then the result of Rutherford's gold foil experiment would have been :
1) All of the α-particles pass through the gold foil without a decrease in speed.
2) α-Particles are deflected over a wide range of angles.
3) All α-particles get bounced back by 180∘
4) α-Particles pass through the gold foil deflected by small angles and with reduced speed.
Solution:
Thomson's model is similar to a Plum pudding model in which the electrons are embedded in a positively charged sphere.
If this model were correct, Rutherford's gold foil experiment would have observed the alpha particles pass through the gold foil deflected by small angles and with reduced speed.
Hence, the answer is the option (4).
Example 2: Assertion: Rutherford's atomic model failed to explain the stability of an atom.
Reason: According to Rutherford's atomic model, electrons revolve around the nucleus in circular orbits, but such an arrangement would lead to the acceleration of electrons and ultimately cause the atom to collapse.
(1) Both assertion and reason are true, and the reason is the correct explanation of the assertion.
(2) Both assertion and reason are true, but the reason is not the correct explanation of the assertion.
(3) The assertion is true, but the reason is false.
(4) The assertion is false, but the reason is true.
Solution:
According to Rutherford's atomic model, the electrons revolve around the nucleus in circular orbits, similar to planets orbiting around the sun. However, the model could not explain why the electrons did not emit energy continuously and eventually fell into the nucleus. In classical mechanics, any charged particle undergoing acceleration emits radiation, which causes the particle to lose energy, ultimately causing it to spiral into the nucleus. This limitation was resolved by the development of quantum mechanics, which explained that the electrons exist in discrete energy levels and not in fixed orbits.
Hence, the answer is the option (1).
Example 3: What are the limitations of Rutherford's atomic model?
1) It failed to explain the stability of an atom.
2) It could not explain the spectra of atoms with more than one electron.
3) It did not provide any information about the arrangement of electrons in the atom.
4) All of the above.
Solution:
Rutherford's atomic model was a significant improvement over the previous atomic models, but it had some limitations. Firstly, it could not explain the stability of an atom. According to classical mechanics, any charged particle undergoing acceleration emits radiation, which causes the particle to lose energy, ultimately causing it to spiral into the nucleus. Secondly, the model could not explain the spectra of atoms with more than one electron. Lastly, the model did not provide any information about the arrangement of electrons in the atom. These limitations were later resolved by the development of quantum mechanics.
Hence, the answer is the option (4).
Example 4:
Electrons in a cathode ray tube have been emitted with a velocity of $1000 \mathrm{~m} \mathrm{~s}^{-1}$ . The number of following statements which is/are true about the emitted radiation is
Given : .$\mathrm{h}=6 \times 10^{-34} \mathrm{Js}, \mathrm{m}_{\mathrm{e}}=9 \times 10^{-31} \mathrm{~kg}$. [JEE Main 2023]
(A) The de Broglie wavelength of the electron emitted is $
666.67 \mathrm{~nm} $ .
(B) The characteristic of electrons emitted depends upon the material of the electrodes of the cathode ray tube.
(C) The cathode rays start from the cathode and move toward the anode.
(D) The nature of the emitted electrons depends on the nature of the gas present in the cathode ray tube.
Solution:
(a)
$\begin{aligned} \lambda & =\frac{h}{\mathrm{mv}}=\frac{6 \times 10^{-34}}{9 \times 10^{-31} \times 1000} \\ & =666.67 \times 10^{-9} \mathrm{~m}\end{aligned}$
(C) The cathode ray starts from the Cathode and moves toward the anode.
Hence, the answer is (2).
Practice More Questions From the Link Given Below:
Conclusion
Based on his findings, Rutherford postulated the atomic structure of the elements. Rutherford's atomic model states that: A positively charged particle was concentrated in a very small volume, which also contained the majority of an atom's mass. He referred to this as an atom's nucleus. Rutherford asserted that atom nuclei are surrounded by negatively charged electrons. The electron that envelops the nucleus travels quickly in a circular pattern. He gave these circular routes names for their orbits.
Also check-
Frequently Asked Questions (FAQs)
The purpose of this experiment was to determine the atom's structure.
Although Rutherford's experiment has potential implications in the present era, it also broadened the inventor's perspective on adjacent subjects including nuclear physics, particle physics, and material science. A device called a particle accelerator, which is based on this experiment, is employed in many different types of research.
A thin gold sheet for the experiment, a 100 nm thin sheet, was used.
The particle which has no charge and has a mass nearly equal to that of a proton is a neutron, and it is present in the nucleus of the atom.
Ernest Rutherford's 1909 experiment on the scattering of alpha particles.
