Wurtz Reaction - Definition, Examples, Limitations, FAQs
Wurtz Reaction Definition: An organic chemical reaction known as Wurtz's reaction occurs when sodium metal is reacted with two alkyl halides in the presence of a solution of dry ether to form a higher alkane as well as a compound that contains sodium and the halogen. Among the most important reactions in organometallic chemistry and organic chemistry for the formation of alkanes is the Wurtz reaction. Sodium and dry ether solution couples two alkyl halides to form a longer alkane chain in this reaction.
Charles Adolphe Wurtz, the French chemist who discovered the aldol reaction, is the name of this reaction. Other metals other than sodium can also be used in the Wurtz reaction to produce alkanes, such as silver, indium, activated copper, zinc, and iron. It is possible for side reactions to occur that lead to the formation of alkenes as a result of this reaction since free radicals are involved. Wurtz-Fitting reactions, which are related to the Wurtz reaction, use aryl halides instead of alkyl halides and are very important names in organic chemistry.
Also read -
Equation of the Wurtz reaction class 12 or Wurtz reaction class 11
The Wurtz reaction equation can be written in the following way:
2R-X + 2Na → R-R + 2Na + X–
With this equation, it becomes apparent that the two R groups combine to yield the alkane with the longer chain coupled with Na X, where X is a Halogen.
Anatomy of the Wurtz reaction
A free radical species R• is involved in the Wurtz reaction and it takes place as part of the halogen-metal exchange. Several mechanisms are involved in this reaction, such as those involving Grignard reagents. A nucleophilic substitution reaction forms the carbon-carbon bond in this reaction mechanism, which is broken down into three steps:
Step 1: An electron is transferred from the metal (in this case, sodium) to the halogen, and a radical is formed along with the alkyl group. It is possible to write this reaction as follows.
R-X + Na → R• + Na + X–
Step 2: The Alkyl radical is now donated an electron by a different sodium atom, leading to the formation of an Alkyl Anion.
R• + Na → R–Na+
Step 3: The carbon of the alkyl anion, which is nucleophilic, displaces the halogen in the alkyl halide via the SN2 reaction and binds with the carbon with which the halogen was bound. Detailed steps of this reaction are outlined below.
Related Topics Link, |
R–Na+ + R-X → R-R + Na +X–
Wurtz reaction involves the production of an alkene as a side product through a free radical mechanism, as we discussed earlier. As shown in the reaction below, this side reaction is explained.
Wurtz's reaction mechanism produces the necessary alkane product. Due to the formation of multiple products, the reaction has relatively low yields.
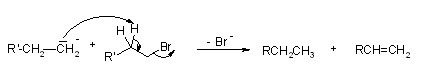
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10 Haloalkanes and Haloarenes
- NCERT notes Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
Wurtz reaction limitations
The following limitations apply to this reaction.
The Wurtz reaction cannot produce methane because the product of an organic coupling reaction must contain at least two carbon atoms.
Using tertiary alkyl halides generally fails the Wurtz coupling method.
Reaction Conditions and Wurtz Reaction Examples
This reaction is rarely used due to several limitations. Various functional groups are intolerant of it, for wurtz reaction example. However, the Wurtz coupling is well-suited to closing small rings, including rings with three members.
This method yields 95% bicyclobutane from 1-bromo-3-chlorocyclobutane. Sodium is liquid at the temperature of refluxing dioxane when the reaction takes place. The multiple products that are formed during this reaction cause the reaction to have poor yields.
The formation of cyclic products occurs in the case of (1,3), (1,4), (1,5), (1,6) dihalides. It forms alkenes in vicinal dihalides but forms alkynes in geminal dihalides.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
