Wavelength of Light - Definition, Calculation, FAQs

What is wavelength of light ?
There is a distance between two consecutive maxima or two consecutive minima in a transverse wave what is called wavelength of light. It is the answer for the question what is wavelength or definition of wavelength of in Physics or Chemistry. Understanding the concept of what is wavelength of helps us in understanding the propagation of light through vacuum. According to famous physicist Maxwell, light is a transverse electromagnetic wave. In understanding the concept of what is wavelength we always follow rules for transverse electromagnetic waves. The wavelength meaning in tamil is “Alianilam”.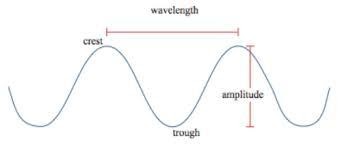
The wavelength of light or visible spectrum wavelength or light spectrum wavelength or the wavelength of visible light ranges up to a certain value of wavelength. Understanding the wavelength of visible or visible light wavelength range or visible spectrum wavelength is very important in optics The visible light wavelength range is approximately 380 to 780 nanometers or visible light spectrum wavelength ranges from 380 to 780 nanometers .The wavelength of red light is approximately 700 nanometer. In Angstrom unit the wavelength of red light is 7000 Angstrom. Although the wavelength of red colour can vary from 620 nanometer to 750 nanometer. Hence in Angstrom unit the wavelength of red light can vary from 6200 Angstrom to 7500 Angstrom. The wavelength of blue light can vary from 450 nanometer to 495 nanometer .In Angstrom unit the wavelength range of blue is 4500 Angstrom to 4950 Angstrom. Wavelength of violet light is around 380 nanometer. In Angstrom unit the wavelength of violet light is 3800. Wavelength of green light is around 550 nanometer. In Angstrom unit the wavelength of green light is 5500 Angstrom. The wavelength of orange is 600 nanometer. In Angstrom unit the wavelength of orange light is 6000 Angstrom. The wavelength of yellow light is 580 nanometer. In Angstrom unit the wavelength of yellow light is 5800 Angstrom. The wavelength of indigo light varies around 425 to 445 nanometer. In Angstrom unit the wavelength of indigo light varies from 4250 to 4450 Angstrom. The conversion formula between Angstrom and nanometer is:-
1 Angstrom = 0.1 Nanometer
This the nm definition in science.
A visible light can be split in to seven colors. These all light colors’ color of light is determined by different wavelengths, frequencies and velocities. These seven colors of visible light are violet, indigo, blue, green, yellow, orange and red. These colors are called VIBGYOR colors. In VIBGYOR wavelength order the colors are defined as : V defines violet, I defines indigo, B defines blue, G defines green, Y defines yellow, O defines orange and R defines red. These colors are called rainbow colors too. The color of light is determined by wavelength and frequency.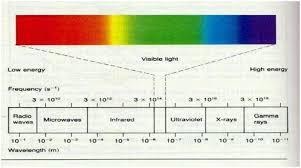
Lambda max definition: The maximum wavelength is called the lambda max.
Red is the color which electromagnetic has the longest wavelength or largest wavelength whereas violet has the lowest or shortest wavelength of light. The wavelengths of the other colored lights fall in between the wavelength of red and violet. The wavelength range for UV spectrum of light is 4× 10-7 to 6× 10-10 meter.
Also read -
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 12 Physics
- NCERT Solutions for All Subjects
Wavelength chart:
| Color wavelengths | Range of wavelength | |
| Radio wave | 104 meter to 0.1 meter | |
| Microwave wave | 0.3 to 10-4 meter | |
| Infrared wave | 10-3 to 7× 10-7 meter | |
| Visible light wave | 7× 10-7 to 4× 10-7 meter | |
| UV spectrum | 4× 10-7 to 6× 10-10 meter | |
| X – ray wave | 10-8 to 10-12 meter | |
| Gamma ray wave | 10-10 to 10-14 meter | |
The wavelength of light is determined by the following formula:-
Wavelength = velocity of light / frequency of the light.
We usually denote wavelength of light by λ , velocity of light by v and frequency of light by f.
Hence wavelength of light can be written as:-
λ= v/f
Lambda max calculation will be vmax/fmin
For maximum wavelength frequency should be minimum and velocity should be maximum. For minimum wavelength frequency should be maximum and velocity should be minimum.
From the above formula we can say that the wavelength of light is dependent on its velocity and frequency.
Light travels slower in glass than air because the refractive index is higher in glass. This happens due to Snell’s law.
Related Topics Link, |
Frequency of light spectrum:-
Frequency of light with different wavelengths are measured in Terahertz. We call Hertz in short Hz and Terahertz in short THz. Frequency is the number of waves passing a point in space during any interval of time, usually one second. We measure frequency in Hertz. One Terahertz is equals to 1014 Hertz.
Frequency of visible light in Hz or range of visible region ranges differently for different colors. Here we will be discussing about different wavelengths of light .Frequency of red light is around 426 Terahertz. Red light has minimum frequency and maximum wavelength. Frequency of blue light is 666 Terahertz. Blue light has maximum frequency and minimum wavelength. As light is an electromagnetic wave, we can say red light is also an electromagnetic wave which has longest wavelength. Blue light is an electromagnetic wave which has lowest wavelength.
Light spectrum definition
It is commonly said as radiant energy which can produce the sensation of vision.
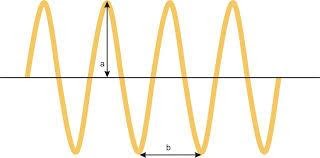
Amplitude of light:
The light waves have different amplitudes. The intensity of the light depends on different amplitudes of light.
Amplitude of light is defined as a.
Wavelength selectors:-
Wavelength selectors absorb the light radiation from a sample. Then the wavelength selector either selects or rejects a certain amount of radiation. Sensitivity of the instrument depends on the bandwidths and detectability depends on transmission.
There are many types of wave length selectors. We are considering filters, grating monochromators and prism monochromators only.
Filters :-
Filters selects wavelength up to narrow bandwidths of radiation. There are 4 types of filters: (i) absorption filter (ii) cut-off filters (iii) interference filters (iv) interference wedges.

Grating monochromators:-
Grating monochromators produce narrow bands of radiation. There are five components in most grating monochromators.
Prism monochromators:-
Light is refracted by the prism at two interfaces. The prism monochromator creates angular dispersion.
What is monochromatic light wavelength?
The light which has only one frequency is called monochromatic light. The corresponding wavelength of that light will be monochromatic light wavelength.
Why light is reffered to as radiation?
According to Maxwell, light is an electromagnetic transverse wave. In
Quantum concept it is considered that light carries energy. Hence light is called as radiation.
Also Read:
- NCERT solutions for Class 12 Physics Chapter 8 Electromagnetic Waves
- NCERT Exemplar Class 12 Physics Solutions Chapter 8 Electromagnetic Waves
- NCERT notes Class 12 Physics Chapter 8 Electromagnetic Waves
What is optics?
Optics is a branch of physics. In optics we study the nature and properties of light. The term light is commonly used to mean a wave of photons which can produce sensation of vision. However now we use the term light to mean all kinds of radiations, both visible and invisible. The subject of optics is divided into three distinct branches on the basis of definations. The three branches of light are (a) Geometrical optics
(b) Physical optics and (c) Quantum optics.
What is Geometrical optics?
In geometrical optics many fundamental principles concerning light are studied by purely geometrical means without assuming anything regarding the nature of light. It assumes rectilinear propagation of light.
What is Physical optics?
In physical optics many experimental results are obtained by considering primarily the wave nature of light.
What is Quantum optics?
In quantum optics one considers the corpuscular nature of light and for exact analysis it requires the method of quantum mechanics.
What is an electromagnetic wave?
According to Maxwell light is electromagnetic wave. A changing magnetic field produces an electric field and a changing electric field in return produces a changing magnetic field. In this way the electromagnetic wave propagates. It propagates through vacuum.
What is UV spectrum light?
The UV light ranges from 4× 10-7 meter to 6× 10-10 meter. The source of this type of light is the sun. The ozone layer in stratosphere absorbs this harmful UV light. The UV light is extremely harmful for us. There are some chemical elements who react with ozone in ozone layer and decreases the density of ozone in ozone layer. These substances are called ozone depleting substance. Many ozone depleting elements are manmade. Like we use ozone depleting elements in aerosol cans and refrigerant. Although many countries are trying hard to ban these types of products.
Also check-
- NCERT Exemplar Class 11th Physics Solutions
- NCERT Exemplar Class 12th Physics Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Physics Notes:
