Boric Acid - Overview, Structure, Preparation, Uses, FAQs
What makes boric acid different from typical mineral acids, and why is it considered a weak acid despite containing three hydroxyl groups? Boric acid chemical formula $\mathrm{H}_3 \mathrm{BO}_3$ and it is a monobasic Lewis acid. It is usually found in powder form. The boric acid powder is also called hydrogen borate, boracic acid, Actium Boricua, or Orthoboric Acid, Antiviral, antifungal, and antiseptic properties make this an effective acid. There is no particular smell associated with boric acid powder since it is soluble in water. A white powdery compound may also exist under standard conditions.
This Story also Contains
- Structure of Boric Acid
- Preparation of Boric Acid
- Properties of Boric Acid
- Medicines Containing Boric Acid
- Uses of Boric Acid
- Poisoning by Boric Acid
- Some Solved Examples

Structure of Boric Acid
- A trigonal planar geometry of oxygen atoms surrounds the boron. O-H is 97 pm long, while B-O is 136 cm long. There are three molecular point groups in the molecule.
- B(OH)3 molecules arranged in layers in crystallized boric acid are linked by hydrogen bonds of 272 micrometres in length. Three hundred eighty-six pixels separate two adjacent layers.
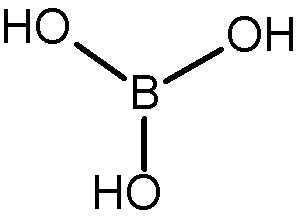
1. Molecular structure
- The formula of boric acid is $\mathbf{H}_3 \mathbf{B O}_3$.
- The boron atom is bonded to three hydroxyl (-OH) groups.
- Each boron-oxygen bond is covalent.
2. Geometry and hybridisation
- Boron is $\mathbf{s p}^2$ hybridised.
- The molecule has a trigonal planar geometry.
- Bond angle is approximately $\mathbf{1 2 0}^{\circ}$.
Preparation of Boric Acid
1. From Borax using Hydrochloric Acid
When an aqueous solution of Borax is treated with hydrochloric acid, boric acid is formed:
$\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7+2 \mathrm{HCl}+5 \mathrm{H}_2 \mathrm{O} \rightarrow 4 \mathrm{H}_3 \mathrm{BO}_3+2 \mathrm{NaCl}$
Explanation:
- Borax reacts with HCl in the presence of water
- Boric acid crystallises out on cooling due to its low solubility in cold water
2. From Borax using Sulphuric Acid
Borax can also be treated with sulphuric acid:
$\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7+\mathrm{H}_2 \mathrm{SO}_4+5 \mathrm{H}_2 \mathrm{O} \rightarrow 4 \mathrm{H}_3 \mathrm{BO}_3+\mathrm{Na}_2 \mathrm{SO}_4$
3. From Boron Trioxide
Boric acid can be prepared by dissolving boron trioxide in hot water:
$\mathrm{B}_2 \mathrm{O}_3+3 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{H}_3 \mathrm{BO}_3$
Also read :
Properties of Boric Acid
| $\mathrm{H}_3 \mathrm{BO}_3$ |
Boric acid |
|
Molar Mass/Molecular Weight |
61.83 g·mol−1 |
|
Density |
1.435 g/cm3 |
|
Boiling Point |
158 °C |
|
Melting Point |
300 °C |
As a crystalline solid, boric acid is soluble in water under standard conditions of temperature and pressure (STP). It is temperature-dependent how well H3BO3 dissolves in water. Solubility is 57 grams per liter of boric acid in water at a temperature of 25 degrees Celsius. This compound is, however, soluble in water when heated to 100 degrees Celsius, where its solubility increases to approximately 275 grams per liter.
Furthermore, boric acid is only slightly soluble in acetone and barely soluble in pyridine. An ion of boric acid is called the borate anion as its conjugate base.
Boric acid solutions are acidic when containing polyols containing cis-vicinal diols (for example, mannitol and glycerol). Under different concentrations of mannitol, pK of B(OH)3 has been found to vary by five orders of magnitude (from 9 to 4). As a result, boric acid with increased acidity can be called magnetologic acid in presence of mannitol.
Medicines Containing Boric Acid
A common use of boric acid in the treatment of minor cuts and burns is as an antiseptic. In addition to its use in dressings and salves, this compound is also used in medicine. You can use boric acid as an eyewash at very dilute concentrations. Humans can also treat acne with boric acid because of its antibacterial properties. It can be found in powdered form. Additionally, it can be sprinkled in socks and shoes to prevent athletes'
Consuming or inhaling excessive amounts of boric acid can lead to poisoning. Boric acid can also seriously damage the kidneys after prolonged exposure.
|
Related Topics |
Uses of Boric Acid
Boric acid is used for the following purposes.
Fiberglass textiles are made with it
-
Inflatable displays are made from this material
-
In hydrofluoric acid, it neutralizes the active acids.
-
A blacksmith uses it to make welding flux
-
The electroplating process uses this.
-
Jewellery manufacturers use it.
-
Silly putty is made from it
-
Insecticidal properties.
-
Antiseptic and antibacterial properties make it useful.
-
As a dry lubricant, it is used on carrom boards.
-
Some nuclear plants use it as a neutron poison
-
It preserves grains like wheat and rice.
-
Boric powder for cockroaches is best for pest control.
The use of boric acid is not uncommon when it comes to the list of chemical additives included in hydraulic fracturing (also known as fracking). Furthermore, it is also used as a cross-linking and gelling agent in the drilling fluid, which is pumped at high pressure into wells, so that its viscosity and rheology can be regulated.
Moreover, it is essential to regulate the fluid viscosity in order to keep the grains of the propping agents suspended for long distances during transportation, as well as to keep the cracks in the shales open for gas extraction after relieving the hydraulic pressure.
Poisoning by Boric Acid
Chronic or acute poisoning from this substance is possible. The effects of acute boric acid poisoning are similar to those of roach-killing powders. When ingested in large quantities, boric acid can cause serious damage to many areas of the body. It may take several weeks for the damage to the esophagus and stomach to resolve after swallowing boric acid. Death may result from complications that manifest months after the initial diagnosis.
Studies have shown that exposure to borax did not cause long-term respiratory problems. However, boric acid is linked to gastrointestinal discomfort over the long term. Common side effects include headaches, fever, tremors, twitching, lack of energy, and weakness. There have been varying limits to the use of borax in insecticides in the United States since 1946. Borax was abolished from all EPA limits in February 1986 due to its low toxicity, according to two EPA records.
Also read -
Some Solved Examples
Question 1: Boric acid is polymeric due to
1) Its acidic nature
2) (correct) The presence of a hydrogen bond
3) Its monobasic nature
4) Its geometry
Solution:
As we have learnt,
Boric Acid has a layer structure in which planar H3BO3 units are linked by H-bonding

Hence, the answer is option (2).
Question 2: When $\mathrm{H}_3 \mathrm{BO}_3$ react with $\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}$ it forms
1) $\mathrm{CH}_3-\mathrm{CHO}$
2) $\mathrm{HBO}_2$
3) (correct) $\mathrm{B}\left(\mathrm{OC}_2 \mathrm{H}_5\right)_3$
4) $\mathrm{B}_2 \mathrm{O}_3$
Solution:
As we have learned
Test for borate radical in qualitative analysis -
When heated with $\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}$ and conc. $\mathrm{H}_2 \mathrm{SO}_4$ gives volatile vapours of triethyl borate which burns with a green-edged flame.
$\begin{aligned}
& \mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7+\mathrm{H}_2 \mathrm{SO}_4+5 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Na}_2 \mathrm{SO}_4+4 \mathrm{H}_3 \mathrm{BO}_3 \\
& \mathrm{H}_3 \mathrm{BO}_3+3 \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH} \rightarrow \mathrm{~B}\left(\mathrm{OC}_2 \mathrm{H}_5\right)_3+3 \mathrm{H}_2 \mathrm{O}
\end{aligned}$
Hence, the answer is option (3).
Question 3: Boric acid is an acid because its molecule:
1) contains replaceable $\mathrm{H}^{+}$ion
2) gives up a proton
3) (correct) accepts $\mathrm{OH}^{-}$from water releasing proton
4) combines with a proton from a water molecule
Solution:
Boric Acid as Lewis Acid - Weak monobasic acid. Does not liberate hydrogen ions but accepts a hydroxyl ion.
$\mathrm{B}(\mathrm{OH})_3+\mathrm{HOH} \rightarrow\left[\mathrm{~B}(\mathrm{OH})_4\right]^{-}+\mathrm{H}^{+}$
Hence, the answer is option (3)
Frequently Asked Questions (FAQs)
It is crippling to consume boric acid. Liquid ammonia is soluble in acetone, glycerol, ether, alcohol, and methanol, but only partially soluble in acetone in water. Sodium borate or halides of boron can be hydrolysed to form boric acid. Boron oxide crystals are somewhat soluble in cold water and somewhat soluble in hot water.
Among its numerous chemical applications are antiseptics, insecticides, flame retardants, neutron absorbents, and precursors.
Acid and base are damaged by neutralization because their acidic properties and fundamental properties are altered. Acids can be neutralized with lime and baking soda, which are both readily available chemicals.
In addition to being an antiseptic, insecticide, flame retardant, neutron absorbent, and precursor to other chemical compounds, boronic acid is also known as hydrogen borate, boracic acid, orthoboric acid, or acid Boricua.
Yes, boric acid can be beneficial in gardening to combat pests. Additionally, it may help with fungal diseases. However, care should be taken to apply it judiciously, as excessive use can harm beneficial insects and the soil ecosystem.
Boric acid serves as an excellent cleaner for all types of mold problems and insects such as ants, cockroaches, silverfish, fleas, and others.
