Borax Formula - Meaning. Formula, Resources, Features, Uses, FAQs
Borax, chemically known as sodium tetraborate decahydrate ($\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7 \cdot 10 \mathrm{H}_2 \mathrm{O}$). It is a white crystalline mineral that is highly soluble in water. It is a boron-sodium-oxygen-water compound and forms a structural geometry that accounts for many of its properties. Borax is essentially an alkaline mineral that serves as a pH buffer, neutralizing acids in many applications.
This Story also Contains
- Borax Formula
- Structure of Borax
- Borax: Various Kinds and Aspects
- Real-World Relevance and Applications of Borax
- Some Solved Examples
Borax Formula
The molecular formula of Borax is $\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7 \cdot 10 \mathrm{H}_2 \mathrm{O}$. Its chemical name is Sodium borate. Where,
- Na is Sodium
- B is Boron
- O is Oxygen,
- H is Hydrogen, and Subscripts indicate the number of atoms of the respective element in the compound.
This is a very old compound, known since ancient times, with ancient civilizations knowing its cleansing and preservative properties. In the present day, major applications of Borax include being a cleaning agent, laundry booster, and pest control solution. It softens water so much that it is used in cleaning applications, which provides the maximum utility of detergents. It is an antifungal and a bactericide, which explains its use in cleaning as well.
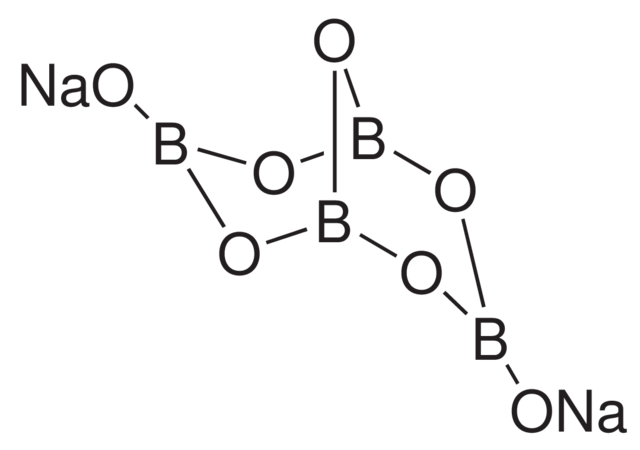
It is the most important compound of Boron. It is a white crystalline solid of formula $\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7 \cdot 10 \mathrm{H}_2 \mathrm{O}$. In fact, it contains the tetranuclear units $\left[\mathrm{B}_4 \mathrm{O}_5(\mathrm{OH})_4\right]^{2-}$and the correct formula; therefore, is. Borax dissolves in water to give an alkaline solution.
$\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7+7 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{NaOH}+4 \mathrm{H}_3 \mathrm{BO}_3$
On heating, borax first loses water molecules and swells up. On further heating, it turns into a transparent liquid, which solidifies into the glass-like material known as a borax bead.
$\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7 \cdot 10 \mathrm{H}_2 \mathrm{O} \xrightarrow{\Delta} \mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7 \xrightarrow{\Delta} 2 \mathrm{NaBO}_2+\mathrm{B}_2 \mathrm{O}_3$
The metaborates of many transition metals have characteristic colors and, therefore, the borax bead test can be used to identify them in the laboratory. For example, when borax is heated in a Bunsen burner flame with CoO on a loop of platinum wire, a blue colored
Co(BO2)2 bead is formed.
Structure of Borax
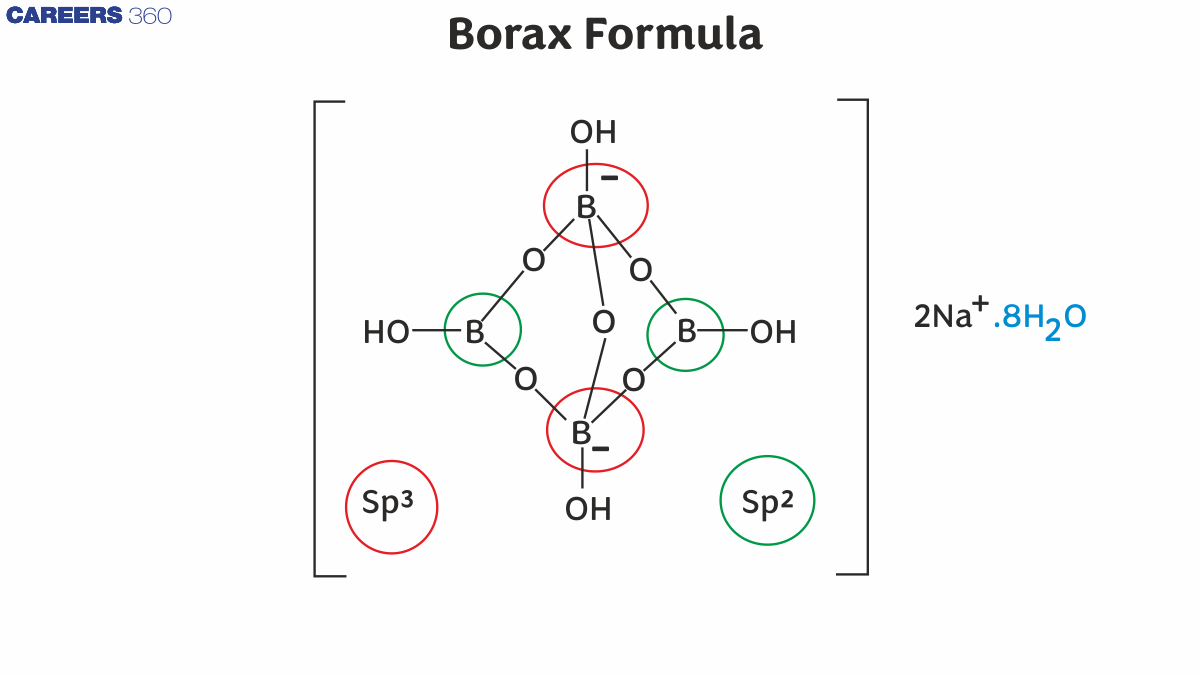
Borax, also known as sodium tetraborate decahydrate, has a polymeric ionic structure in the solid state.
1. Ionic nature
- Borax consists of $2 \mathrm{Na}^{+}$ions and the tetraborate anion $\left(\mathrm{B}_4 \mathrm{O}_7^{2-}\right)$.
- The sodium ions balance the -2 charge of the tetraborate ion.
2. Tetraborate ion $\left(\mathrm{B}_4 \mathrm{O}_7^{2-}\right)$
- The $\mathrm{B}_4 \mathrm{O}_7{ }^{2-}$ ion is formed by the condensation of two $\mathrm{BO}_3$ (trigonal planar) units and two $\mathrm{BO}_4$ (tetrahedral) units.
- These units are linked through bridging oxygen atoms (-O-).
- Thus, boron shows both $\mathrm{sp}^2$ and $\mathrm{sp}^3$ hybridisation in borax.
3. Water of crystallisation
- Borax contains 10 molecules of water.
- 8 molecules are loosely bound as water of crystallisation.
- $\mathbf{2}$ molecules are directly linked to boron via hydrogen bonding.
- Water molecules help maintain the crystal lattice stability.
4. Overall structure
- The structure can be visualised as:
- A central $\mathrm{B}_4 \mathrm{O}_7{ }^{2-}$ framework
- Surrounded by $\mathrm{Na}^{+}$ions
- Stabilised by water molecules through hydrogen bonding
Borax: Various Kinds and Aspects
Though most people may only be familiar with the common, powdered form of Borax, in reality, there are a number of types and formulations of the mineral to best serve its purpose. One of the most common forms is that of the decahydrate, which physically holds ten molecules of water; however, the Borax also exists in anhydrous forms that do not contain water and are primarily used for industrial applications.
Apart from this, Borax is also available in numerous combinations and products that are manufactured for numerous performances. For instance, a common constituent of most laundry detergents is Borax—a water softener and stain remover. It's an element of the cream that provides cleaning of mold and mildew—fully exploiting its antifungal features. In DIY projects, Borax is highly utilized in the making of slime, and it is a fun activity to have kids involved since it's a learning process; it introduces simple chemical reactions.
It is observed that borax is not just any other household substance; the mineral plays an active role in agriculture as one of the micronutrients for plants in boron-deficient soils. That explains how high, in terms of importance, the compound is, and how versatile it high in both domestic activities and commercially oriented areas.
Related Topics
Real-World Relevance and Applications of Borax
The many real-world applications of Borax range from household cleaning to scientific research. Many people rely on Borax as their partner in cleaning their houses. After all, it can be added to laundry to make it more efficient and actually possesses qualities that make it an excellent stain remover and odor eliminator while softening water. One of the most preferred alternatives for households with eco-friendly cleaning solutions is to just mix Borax with water to create enough solutions that can clean off heavy dirt on surfaces.
Borax is a very promising insecticide when it comes to pest control against cockroaches and ants. This will dehydrate their digestive systems upon consumption and thus kill the pests that way. Favoritism is thus done with the fact that this kind of pest control is more favorable compared with those that are chemically based; thus, Borax is often the thing when one wishes to curb exposure to hazardous chemical substances.
This variety is widely used at school to explain loaded concepts, such as solubility, pH, and chemical reactions. This is because the variety is not dangerous and is considered the perfect variant to execute educational experiments. For example, making slime with Borax and glue is a cool way for children can access polymer chemistry and the qualities of non-Newtonian fluids.
Recommended topic video on (Borax Formula)
Also read -
Some Solved Examples
Example 1: When Borax is strongly heated it appears as
1) opaque glassy bead on cooling
2) (correct) transparent glassy bead on cooling
3) white needle-shaped crystal
4) blue glassy bead on cooling
Solution:
As we have learnt,
Borax bead -
Used for the detection of coloured basic radicals under the name borax bead test.
$\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7 \cdot 10 \mathrm{H}_2 \mathrm{O} \xrightarrow[-\mathrm{H}_2 \mathrm{O}]{\Delta} \mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7 \xrightarrow{\Delta} 2 \mathrm{NaBO}_2+\mathrm{B}_2 \mathrm{O}_3$ 
A transparent Glassy bead is obtained which is called borax bead.
Hence, the answer is option (2).
Example 2: Which of the following ions can be detected by the borax bead test?
1) Ni⁺
2) Co²⁺
3) Pb²⁺
4) Both a and b (correct)
Solution: The borax bead test is used to identify colored basic radicals, specifically transition metals. Both nickel (Ni²⁺) and cobalt (Co²⁺) can be detected using this method.
Therefore, the correct answer is option (4).
Example 3: What is the color of cobalt metaborate?
1) Blue (correct)
2) Brown
3) Pink
4) Red
Solution: Cobalt metaborate, formed during the borax bead test, exhibits a distinct blue color.
Hence, the correct answer is option (1).
Example 4: What are the products formed when a given quantity of sulphuric acid is treated with an aqueous solution of borax?
1) $\mathrm{H}_3 \mathrm{BO}_3, \mathrm{NaHSO}_4$
2) (correct) $\mathrm{Na}_2 \mathrm{SO}_4, \mathrm{H}_3 \mathrm{BO}_3$
3) $\mathrm{Na}_2 \mathrm{BO}_3, \mathrm{H}_3 \mathrm{BO}_3$
4) None
Solution:
$\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7+\mathrm{H}_2 \mathrm{SO}_4+5 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Na}_2 \mathrm{SO}_4+4 \mathrm{H}_3 \mathrm{BO}_3$
Hence, the answer is option (2).
Example 5: In the borax bead test, which of the following compounds is formed?
1) (correct) Meta borate
2) Tetra borate
3) Double oxide
4) Ortho borate
Solution:
When borax is heated, we obtain Sodium Metaborate and boric oxide.
$\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7 \cdot 10 \mathrm{H}_2 \mathrm{O} \xrightarrow[-10 \mathrm{H}_2 \mathrm{O}]{ } \mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7 \xrightarrow{\Delta} 2 \mathrm{NaBO}_2+\mathrm{B}_2 \mathrm{O}_3$
Boric oxide reacts with metal oxides and forms the metal metaborate
$\mathrm{CuO}+\mathrm{B}_2 \mathrm{O}_3 \rightarrow \mathrm{Cu}\left(\mathrm{BO}_2\right)_2$
(Copper meta borate)
Thus, metaborates of the metal are obtained in the Borax bead test.
Hence, the answer is option (1).
Practice More questions with the link given below
Frequently Asked Questions (FAQs)
Borax, also known as sodium borate, has the chemical formula (Na2B4O7⋅10H2O). This indicates that it is comprised of sodium (Na), boron (B), oxygen (O), and water (H2O).
Borax is used for the safekeeping, transport, and use of radioactive material in neutron-capture shields. This compound is also known to act as an antifungal agent, so it can be used to destroy mold or prevent its growth.
The field of biochemistry is known for its extensive use of borax to produce buffer solutions.
While some studies suggest that borax is nontoxic, it can be noted that this compound is highly toxic to insects and is a potent insecticide. It can also be noted that borax is a potential carcinogen and can increase the risk of liver cancer. In addition, repeated exposure to large amounts of borax can cause irritation to the respiratory system.
Baking soda and borax are obtained by combining many widely available chemicals. Borax is classified as white borate white, while baking soda is classified as white crystal bicarbonate.
When borax is added to a flame, it produces a yellow-green color. Borax is not used for this purpose in fireworks due to the overwhelming yellow color of sodium