Colorimeter - Beer’s Law, Lambert’s Law, Principle, Parts, Applications, FAQs
Colorimetry is a scientific technique that employs the Beer–Lambert rule to determine the concentration of coloured substances in solutions. A colorimeter is a colorimetry tool.It refers to a device that aids in the absorption of a given wavelength of light by specific solutions. It measures absorbance and wavelength between 400 and 700 nm (nanometer), i.e. from the visible spectrum of light. Using the Beer-Lambert rule, the colorimeter is often used to determine the concentration of a known solute in a given solution. In the year 1870, Louis J Duboscq devised the colorimeter
This Story also Contains
- Working principle of colorimeter
- Beer’s Law
- Lambert’s Law
- Parts of Colorimeter
- Working of Colorimeter
- Applications of Colorimeter
- Colorimeter v/s Spectrophotometer
Working principle of colorimeter
Let as discuss the working principle of the colorimeter.The photometric technique is used in a colorimeter asserts that when a beam of incident light of intensity (I0) passes through a solution, a portion of it is reflected (Ir), absorbed (Ia), and the rest is transmitted (It)
I0 =Ir +Ia+It
Because of the measurement of (I0), (Ir) is deleted in colorimeters, and it is sufficient to determine the value of (Ia). This is accomplished by utilising cells with equal characteristics to keep the amount of light reflected (Ir) constant. After that, (I0), and (It), are calculated.
Colorimeter is based on two basic laws of photometry, which show the mathematical relationship between amount of light absorbed and the concentration of the substance.
Also read -
Beer’s Law
The amount of light absorbed is proportional to the amount of solute in solution, as per Beer's law.
Log10(I0/It)=as c
Where as=Absorbency index
c =Concentration of the solution
Lambert’s Law
According to Lambert's law, the amount of light absorbed and thickness or length of the solution are directly proportional
A = Log10(I0/It)= asb
Where,
A = Absorbance of the solution
as= Absorbancy index
b = thickness of the solution
Parts of Colorimeter
A colorimeter has five key components.
Light Source
A tungsten filament is the most popular light source in colorimeters.
Monochromator
Filters or monochromators are used to separate the light from the light source to select a specific wavelength.
Sample holder
Color solutions are held in test tubes or cuvettes, which are composed of glass at visible wavelengths.
Photodetector system
An electric current is generated when light falls on the detection system, which reflects the Galvanometer reading.
Measuring device
The current from the detector is routed into the Galvanometer, which displays a metre reading that is directly proportional to light intensity.
Colorimeter diagram
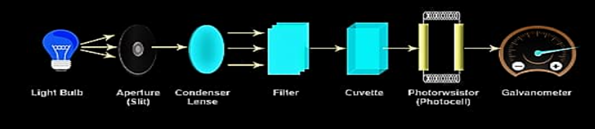
Working of Colorimeter
There are five steps involved in the working of colorimeter
It is necessary to calibrate the colorimeter before beginning the experiment. It's done with the help of standard solutions containing the known solute concentration to be determined. Fill the cuvettes with standard solutions and set them in the colorimeter's cuvette holder.
A light ray of a specific wavelength for the assay is sent in the direction of the solution. The light is filtered through a succession of lenses and filters. Colored light passes through lenses and the filter splits a beam of light into different wavelengths so that only the required wavelength can pass through the standard test solution in the cuvette.
The laser beam is transmitted, reflected, and absorbed by the solution when it reaches the cuvette. The photodetector device monitors the intensity of transmitted light when the transmitted ray hits it. It turns the data into electrical impulses, which it then sends to the galvanometer.
The galvanometer's electrical signals are shown in a digital format. The absorbance or optical density of the investigated solution is the digital representation of the electrical signals. If the solution absorbs more light, more light is absorbed by the solution, and if the solution absorbs less light, more light is transmitted through the solution, which determines the galvanometer reading
Formula for determining the concentration of a chemical in a test solution.
A = ∈cl
For standard and test solutions
∈ and l are constant
AT= CT
AS= CS
From these equations,
AT× CS= AS× CT
CT= (AT/AS) × CS
Where,
CT= Concentration of the test solution
AT= Absorbance/optical density of test solution
CS= Standard concentration
AS= Absorbance / optical density of standard solution
Also Read:
Applications of Colorimeter
The colorimeter is a device that measures the optical density or absorbance of a coloured chemical to determine its concentration
A substance can be identified using a colorimeter by analyzing its absorption spectra in the visible area of the light spectrum.
It can also be used to determine the reaction's course by observing the rate of creation and disappearance of the light-absorbing compound in the visible spectrum of light.
It is utilized to calculate biochemical samples including urine, cerebrospinal fluid, plasma, and serum in laboratories and hospitals.
It is utilized in the production of paint.
It can be found in the textile and food industries.
It is used to analyze proteins, glucose, and other biological components quantitatively.
It is used to determine the quality of water.
It is used to figure out how much haemoglobin is in your blood.
The main advantage of colorimeter is that it is a low-cost method that is commonly employed in the quantitative examination of coloured samples, as well as one that is simple to carry and transport. The disadvantage is that it is not feasible to analyze colourless chemicals, and it does not work in the IR or UV regions.
Colorimeter v/s Spectrophotometer
The spectrophotometer consists of moving parts, is heavier, and is only suitable for laboratory use. The colorimeter consists of fixed parts and is lighter and therefore suitable for use in the field.
The spectrophotometer measures the amount of light that passes through a sample. Colorimeter measures light absorption.
The wavelength selector of the spectrophotometer is monochromator, wavelength range. The wavelength selector on the colorimeter is a color filter with a fixed wavelength.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
