Distillation - Definition, Examples, Types, Equipments, Advantages
Distillation refers to the selective boiling and subsequent condensation of a portion of the liquid. It is a method of differentiation that can be used to increase the concentration of a particular component in a component or to obtain (almost) pure material components. The beverage process uses a difference in the boiling points of substances in parts of a liquid by forcing one of them to form a gas. It is important to note that distillation is not a chemical reaction but can be considered a physical separation process. The distillation apparatus is commonly called a still.
This Story also Contains
- Boiling Point of Distillation
- Types of Distillations
- Equipment Used in Distillation
- Steam Distillation
- Short Distance Distillation
- Important Applications of Distillation
- Fractional Distillation
- Vacuum Distillation
- Advantages of Vacuum Distillation
- Some Solved Questions
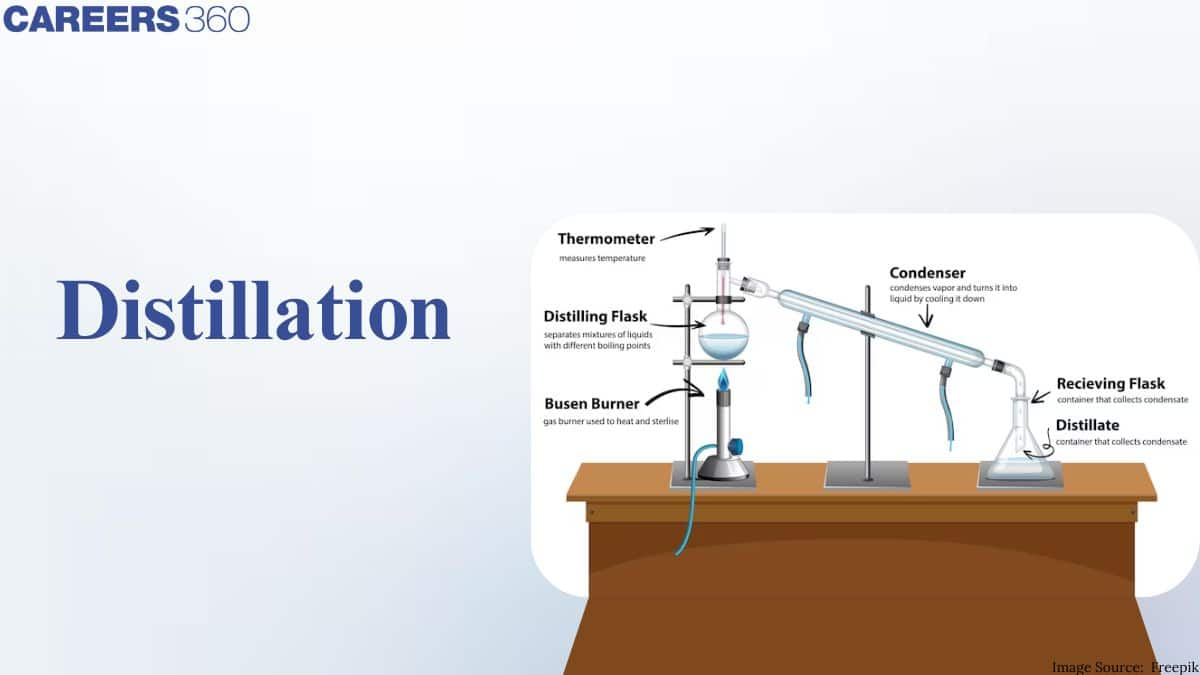
Distillation performed on a laboratory scale usually uses liquid compounds, and the beverage processes are usually filtered continuously, requiring a stable composition of the compound to be maintained. A distillery is a factory where strong alcoholic drinks are produced by the process of distilling.
Boiling Point of Distillation
It is important to note that the boiling point of the liquid changes with the surrounding pressure. For example, the point of boiling water in the ocean is 100 °C, but the boiling point of 1905 meters is 93.4°C (because atmospheric pressure is much lower at higher altitudes). With a combination of beverages, the beverage process is based on Dalton's law and Raoult's law. According to Raoult's law, the absolute pressure of a single liquid in a positive part of a liquid is equal to the product of the vapor pressure of the pure substance and its mole fraction.
According to Dalton's partial pressure law, the total pressure generated by a gas mixture is equal to the sum of pressures that are part of all available gases. When the beverage mixture is heated, the vapor pressure of each component increases, which in turn increases the pressure of total evaporation. Therefore, the mixture may not have as many boiling points in a given composition and pressure.
Types of Distillations
1. Simple Distillation
2. Fractional Distillation
3. Steam Distillation
4. Vacuum Distillation (Reduced Pressure Distillation)
5. Azeotropic Distillation
Equipment Used in Distillation
1. Distillation Flask (Round-Bottom Flask)
- Contains the liquid mixture to be distilled
- Made of borosilicate glass to withstand high temperatures
- Provides uniform heating
2. Heat Source
- Supplies energy to vaporize the liquid
- Commonly used:
-
Bunsen burner
-
Heating mantle
-
Hot plate
3. Thermometer
- Measures the temperature of vapour
- Helps identify the boiling point of the distilling component
- Placed at the mouth of the distillation flask
4. Fractionating Column (used in fractional distillation only)
- Increases surface area for repeated condensation and vaporization
- Ensures better separation of liquids with close boiling points
- Packed with glass beads or plates
5. Condenser (Liebig Condenser)
-
Converts vapour into liquid by cooling
-
Cold water flows in from the bottom and out from the top
-
Common types:
- Liebig condenser
- Graham condenser
- Allihn condenser
6. Receiver / Receiving Flask
- Collects the condensed liquid (distillate)
- May be a conical flask or round-bottom flask
7. Adapter (Distillation Adapter / Receiver Adapter)
- Connects the condenser to the receiving flask
- Directs the flow of condensate
8. Vacuum Adapter (in vacuum distillation)
- Maintains reduced pressure
- Prevents backflow of liquid
9. Pressure-Reducing Equipment (for vacuum distillation)
- Vacuum pump or aspirator
- Lowers boiling point of liquid
10. Steam Generator (for steam distillation)
- Produces steam separately
- Steam passes through the mixture
11. Stand, Clamp, and Rubber Tubing
- Support and secure apparatus
- Rubber tubes supply cooling water to the condenser
Also read :
- NCERT notes Class 12 Chemistry Chapter 6 General Principles and Processes of isolation of elements
- NCERT solutions for Class 12 Chemistry Chapter 6 General Principles and Processes of isolation of elements
- NCERT Exemplar Class 12 Chemistry solutions Chapter 6 General Principles and Processes of isolation of elements
Steam Distillation
- Steam distillation is often used to separate heat-sensitive components in an organization.
- This is done by transferring steam to a mixture (slightly heated) to heat one of them. This process establishes a high degree of heat transfer without the need for high temperatures.
- The lead vapor is condensed to pay for the required distillate.
- The steam distillation process is used to obtain essential oils and herbal distillates from flowers / many fragrant herbs.
- The beverage machine was filtered
- Vacuum distillation is ideal for separating beverage mixtures with highly boiling points.
- To boil these compounds, heating to high temperatures is the wrong approach. Therefore, environmental pressure is reduced instead.
- Reducing pressure causes the component to boil at lower temperatures. When the vapor pressure is equal to the surrounding pressure, it is converted to steam.
- These vapors are trapped and collected as a distillate. Beverage enhancement method is also used to obtain high-quality samples of chemical decomposition at high temperatures.
- Reduced Breathing Air Purifier
- For air-sensitive and easy-to-react chemicals, the beverage process is performed but the vacuum must be replaced with incoming gas once the process is complete. Such a procedure is often called vacuum distillation.
Short Distance Distillation
Short-distance distillation is used to clean a small amount of an unstable mixture at high temperatures. This is done under reduced pressure levels and usually involves a distillate that travels a very short distance before collection (hence the term ‘short way’). The reduced distance traveled by the distillate in this way also reduces the damage along the walls of the appliance.
Local distillation process involves partial melting of the substance and narrowing of the lungs leading to the acquisition of pure distillate. This is done in a long container with the help of a heater zone.
Important Applications of Distillation
The method of drinking alcohol has a long history, dating back to 3000 BC. Evidence suggests that the use of alcohol was widespread in the ninth century. Some important distillation applications are listed below.
- Drinking water plays an important role in many water purification techniques. Many sugar plants use this method to obtain drinking water from seawater.
- Refined water has many applications, such as lead-acid batteries and low-volume humidifiers.
- Many boiled products such as alcoholic beverages are purified with the help of this method.
- Many fragrances and flavors are found in herbs and plants through distillation.
- Oil stabilization is an important type of beverage that reduces the pressure of crude oil vapor, which allows for safe storage and mobility.
- The air can be divided into nitrogen, oxygen and argon through a cryogenic distillation process.
- Distillation is used in various industrial scales to purify liquid products that are derived from chemical compounds.
Fractional Distillation
Fractional distillation is the separation of a mixture into its components or subdivisions. Chemical compounds are separated by heating them until they reach a temperature where one or more part of the mixture will evaporate. It uses distillation to separate. The parts of the parts usually have boiling points that vary below 25 ° C (45 ° F) to each other under the same air pressure. If the difference in boiling points is greater than 25 ° C, a simple distillation is commonly used.
Vacuum Distillation
The beverage machine was filtered
The principles of vacuum distillation are very similar to fractional distillation (commonly it is referred to as atmospheric distillation to separate them from the vacuum method), except larger-sized columns that are used to maintain vapor velocities by reducing operational pressure.
A 50 to 100 mm mercury absolute machine is produced by a suction pump or steam ejector.
Advantages of Vacuum Distillation
The main advantage of vacuum distillation is that it allows the removal of heavy objects at lower temperatures than would be necessary for atmospheric pressure, thus avoiding the hot cracks in the material. Firing conditions in the furnace are adjusted so that the oil temperature generally does not exceed 425 ° C (800 ° F). Residues left behind after vacuum distillation, called bitumen, may be compacted to produce road asphalt or residual oil, or they can be used as a feed for hot cracks or cooking units. Beverage improvement units are an integral part of many processing systems designed to produce cosmetics.
Why is it impossible to Completely Clean the mixture with Distillation?
Where a mixture of beverages boils, all flammable substances are boiled. However, the quantity of an effective combustible substance is derived from its contribution to the absolute vapor pressure of the compound. That is why compounds with high pressure can concentrate on souls, and compounds with low pressure can partially concentrate on fluids. Since the component in the compound may not have an absolute pressure, it is not possible to obtain a completely pure sample of the component from the compound by distillation. However, high-purity samples can be obtained when one of the component parts has a component pressure close to zero.
Also read -
Some Solved Questions
Question 1: Which of the following methods is used for the purification of organic compound to separate volatile liquids from non-volatile impurities?
1) Chromatography
2) (correct) Distillation
3) Crystallisation
4) None of these
Solution:
As we have learnt,
The distillation technique is used to separate a volatile liquid from non-volatile impurities.
Hence, the answer is the option (2)
Question 2: Which one of the following purification technique is used for the separation of a mixture containing acetone and methanol?
1) Distillation
2) Crystallisation
3) Sublimation
4) (correct) Fractional distillation
Solution:
As we have learnt,
Simple distillation is used for the separation of liquids having a significant difference in their boiling points. If the difference in the boiling points is not much, then Fractional distillation is used as the preferred method of separation.
Methanol boils at $65^{\circ} \mathrm{C}$ while acetone boils at $60^{\circ} \mathrm{C}$ .
So, for their separation, we require Fractional Distillation.
Hence, the answer is the option (4).
Question 3: A flask contains a mixture of isohexane and 3-methylpentane. One of the liquids boils at $63^{\circ} \mathrm{C}$ while the other boils at $60^{\circ} \mathrm{C}$ . What is the best way to separate the two liquids and which one will be distilled out first?
1) (correct) Fractional distillation, isohexane
2) simple distillation,3-methylpentane
3) fractional distillation, 3-methylpentane
4) simple distillation, isohexane
Solution:
As we have learned,
Simple distillation is used for the separation of liquids having a significant difference in their boiling points. If the difference in the boiling points is not much, then Fractional distillation is used as the preferred method of separation.
A liquid having a lower boiling point is distilled first in fractional distillation.
So, it is isohexane which is distilled first as it has a lower value of boiling point.
Hence, the answer is option (1).
Question 4: The mixture of benzene and toluene can be separated by -
1) Simple distillation
2) (correct) Fractional distillation
3) separation by the funnel method
4) None
Solution:
As we learned in the concept
Distillation -
Method for separating a mixture of two liquids where the difference in boiling points is large.
wherein
The boiling point increases as the liquid mixture gets enriched in the less volatile liquid
Benzene and toluene are separated by fractional distillation because the difference in their boiling point is very small.
Hence, the answer is the option (2).
Practice more questions with the link given below:
Frequently Asked Questions (FAQs)
Distillation means a selective boiling and subsequent solidification of a portion of the liquid.
The process is called steam distillation when water is used as one of the soft drinks. It is often used to clean liquids that, in their normal boiling point, rot. To remove organic compounds from plant fragments, steam distillation is used.
This behavior occurs because the boiling of a substance requires a low vapor pressure, which may be received at low temperatures. Steam distillation is similar to basic distillation, the only difference being that, with the substance to be added, steam (or water) is used in the drinking pot.
A delicious beverage that separates the intended product from other substances or impurities also cleanses organic compounds. Under the conditions for drinking steam beverages, nitrophenol distils, so o-Nitrophenol is a flexible steamer.
The most common way to remove aromatic chemicals (essential oil) from a plant is by steam distillation. Smoke travels through plant material during the steam distillation process. The combination of hot steam and gentle pressure allows small protective bags to release essential oil.
O-Nitrophenol forms an intramolecular H bond and the P-Nitrophenol molecules bind to intermolecular H bond bonds. During fermentation, strong intermolecular H bonding increases the boiling point but intramolecular H bonding is unable to do so. Therefore, O-Nitrophenol is as versatile as P-Nitrophenol.
Questions related to
On Question asked by student community
Correct Answer: burning Deuterium in oxygen
Solution : The correct option is the burning of deuterium in oxygen.
Heavy water, or deuterium oxide (D2O), is produced by burning deuterium in oxygen. Burning deuterium (a heavy isotope of hydrogen) in oxygen-containing air can result in the production of
