Thomsons Model - J.J Thomson Atomic Theory and FAQs
J.J Thomson biography
Sir Joseph John Thomson was born on 18 December in the year 1856, in Cheetham hill, Manchester, England. J.J Thomson is a well renowned British physicist and a Nobel laureate, who won a Nobel prize among many accolades for the discovery of electrons in the year 1906.
J.J Thomson life
Joseph John Thomson, popularly known as J.J Thomson was a British physicist and a Nobel laureate. Thomson’s father was a bookseller who wanted Thomson to become an engineer. After many trials, Thomson failed to get an apprenticeship at the engineering firm, which led him to Owen’s college back again when he was 14 years old. Thomas received a small scholarship to study mathematics in Trinity College, Cambridge in the year 1876.
After completing his graduation, Thomson worked in the Cavendish laboratory under the guidance of Sir Rayleigh. While working, Thomson immediately earned Royal Society membership, and was appointed as a successor of Rayleigh as the physics professor in Cavendish, when he was 28 years old. He was very much liked and cherished from students all around the globe.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
J.J Thomson atomic theory
The discovery of electron led by Sir J.J Thomson thoroughly change the view of peoples about the atoms, which were thought to be smallest spheres till the 19th century but after which Thomson proposed atomic model theory in the year 1903 which states the atom comprises of negative and positive charges which are in the same concentration making the atom electrically equivalent or neutral.
Thomson proposed the model in the context of chocolate chip cookies or a watermelon and more precisely plum pudding, consisting of an atom as a sphere with the negative and positive charges embedded within. Later Thomson’s atomic models were given a name of Chocolate Chip Model or a Plum Pudding model.
Right now, in this century, researchers know that the nucleus of an atom comprises of neutral charged neutrons and positively charged protons with negatively charged electrons located outside the nucleus into the orbit, still Thomson’s atomic model is important enough because Thomson was the first who point out the charged particles present in the atom.
Explain thomson model of atom
- Before the discovery of electrons by Sir J.J Thomson, researchers used to think that an atom was the smallest and indivisible unit of matter.
- When Thomson first found out the electrons, he named them as corpuscles instead of electrons.
- The mass spectrograph was first invented by Sir Thomas while he discovered the nature of radioactivity of positively charged particles.
Postulates of J.J Thomson model of an atom
- Atoms comprise neutral charge, that is they are electrically neutral.
- The negative charge of electrons is neutralized by the source of positive charge present in the atom.
- The positive charge is thoroughly distributed throughout the atom.
- The electrons can easily get carried along inside of the atom.
- The negatively charged electrons or the negatively charged corpuscles as Thomson mentioned are occupied within the consistent mass of positive charge.
- As the statement given by Gaussian law that the electrons occupy stable orbits, as the electrons shift through the mass positively, the forces present internally inside the electrons are balanced by the positive charges that are instantaneously given throughout the orbit.
- Sir J.J Thomson’s atomic model is well received in England as the Plum Pudding model as the distribution of electrons as discovered by Thomson was identical to the arrangement of plums inside the pudding.
Related Topics Link, |
Thomson plum pudding model
Plum pudding theory
Plum pudding model summary
Sir Thomson, in the year 1897 discovered electrons and proposed the plum pudding’s model of the atom prior to the discovery of the nucleus of the atom in the year 1904, to include electrons in the model of atom.
In the plum pudding model, the corpuscles as mentioned by Thomson are nothing but electrons surrounded by a slurry of positive charge to balance the negative charge of the corpuscles, in the context of plums charged negatively surrounded by the pudding throughout acting as the positive charge.
The electrons are situated around the circular orbit.
This model is also called the watermelon model of an atom because it resembles the watermelon as the atom as whole and seed being positive and negative charges embedded inside.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 2 Structure of Atom
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 2 Structure of Atom
- NCERT notes Class 11 Chemistry Chapter 2 Structure of Atom
Limitations of plum pudding model
- The origin of spectral lines of hydrogen atoms are not explained by Thomson’s plum pudding model.
- It does not take into consideration the large number of alpha particles scattering as noted in Rutherford’s scattering experiment.
- The stability of Thomson’s plum pudding model is zero.
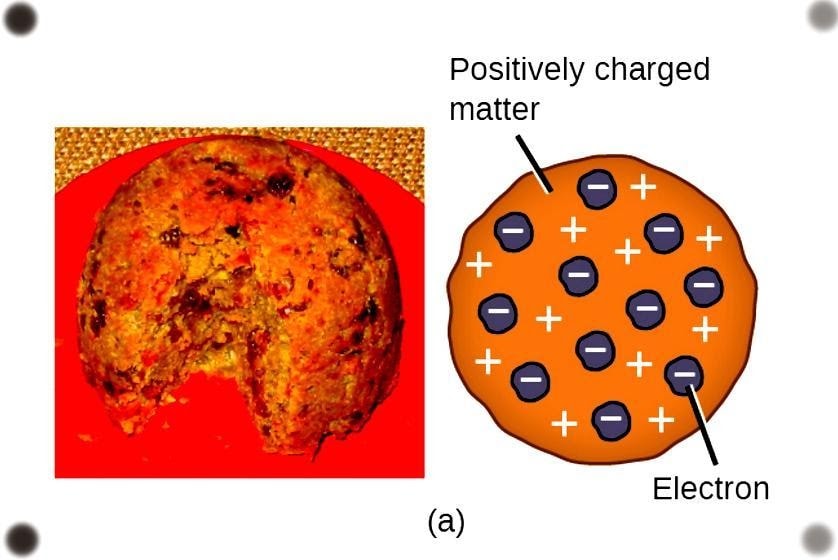 Plum pudding labelled
Plum pudding labelled
Thomson scattering
Thomson scattering is an electromagnetic phenomenon in which elastic scattering of electromagnetic waves occurs. In this phenomenon, the particle's frequency of photon and kinetic energy do not get altered, during the process of scattering. In the scattering, the magnetic and electrical constituents of the incident wave cause the acceleration of the particle in motion. While in the process of acceleration, the particle gives out radiation causing the wave to be scattered. The phenomenon of Thomson scattering is of immense importance in the field of plasma physics.
Sir J.J Thomson first discovered and invented the phenomenon of scattering, thus the name Thomson scattering. The particle in motion will move along in the direction of the electric field, resulting in electromagnetic radiation. This phenomenon is only valid as long as the mass energy is quite bigger than the photon energy of the particle in motion.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
