Bohrs Model - History, Structure, Theories, Limitations, FAQs
Have you ever wondered why electrons in an atom do not spiral into the nucleus despite being attracted to it, or how atoms produce distinct line spectra instead of a continuous range of energies? You will find this answer by Bohr's model of the atom. It was originally proposed by Neil Bohr in 1915. And further modified by Rutherford. The Rutherford model introduces the nuclear atom model, in which he explains that poorly charged electrons surround the nucleus (well-charged). The Bohr model consists of a small (well-charged) nucleus surrounded by negative electrons moving around the nucleus in pathways. Bohr found that electrons located far from the nucleus are more powerful, and electrons close to the nucleus are less powerful.
This Story also Contains
- Postulates of Bohr Model Atom
- Limitations of Bohr Model Atom
- History of the Bohr Model
- Modified Bohr Model
- Some Solved Examples
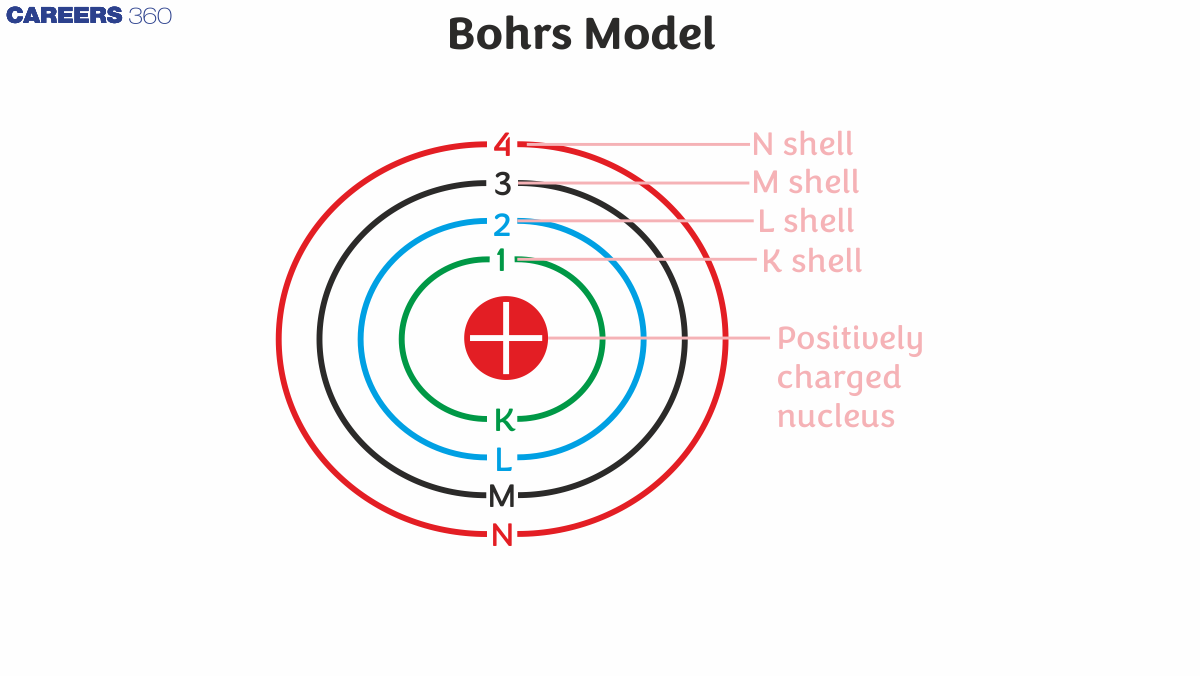
Postulates of Bohr Model Atom
- In an atom, electrons (badly charged) revolve around a well-charged nucleus in a clear circular pattern called orbits or shells.
- Each circle or shell has a fixed force and these circular lines are known as orbital shells.
- Power levels are represented by a whole number (n = 1, 2, 3…) known as a quantum number.
- This quantum numerical range starts from the side of the nucleus with n = 1 having the lowest energy level.
- Orbits n = 1, 2, 3, 4, and so on... are assigned as K, L, M, N, and so on… shells, and when the electron reaches a very low level of energy, it is said to be in the ground state.
- Electrons in the atom go from low energy to high energy by getting the energy needed and electrons move from high energy to high energy levels by losing energy.
Limitations of Bohr Model Atom
- Bohr's atomic model failed to explain the effect of Zeeman (the effect of magnetic force on a series of atoms).
- It also failed to explain the effect of Stark (the effect of the electric field on the atomic spectrum).
- It violates the Heisenberg Uncertainty Principle rule.
- Could not define spectra obtained from large atoms.
Also read :
History of the Bohr Model
The discovery of electrons and radioactivity in the late 19th century led to a variety of proposed atomic formations.
In 1913, Niels Bohr proposed the idea of a hydrogen atom, based on the quantum assumption that a certain body part takes only different amounts. Electrons revolve around the nucleus, but only in fixed lines, and When electrons cross the low-energy path, the difference is sent as radiation. Bohr's model explained why atoms only emit light at fixed wavelengths, and later inject ideas into light quanta.
Modified Bohr Model
Despite the success of the Bohr model, there have been major flaws. On the test side, a detailed analysis of hydrogen extraction found a single extraction line actually consisting of two or more closely spaced lines, a feature not found in the Bohr model. Theoretically, the Bohr model mixes particle images with electron waves, which were considered by many to be unsatisfactory.
For these reasons, better treatment of hydrogen atoms was bought when the electron was considered a wave from the beginning. This theory, developed by Heisenberg, Pauli, Schrödinger, Sommerfeld, and others, is well mathematically accurate. The main result from this theory is that four Quantum Numbers describe the state of the electron, compared to the single quantum n number present in the Bohr model. These quantum numbers are as follows:
- n, the principle quantum number. This is similar to the Bohr type, and can take up values of 1,2,3, ....
- l, orbital quantum number. This is a label that describes the magnitude of the angular pressure of an electron. With a given n, l can take values 0,1,2, ..., n - 1.
- ml, the number of spin-orbital quantum. This is a label that describes the angular component of an electron vector. For a given l, ml can take values - l, - l + 1, ..., - 1,0,1, ..., l - 1, l.
- ms, the quantum spin number. This label, in a very limited sense, can be regarded as setting a point where an electron rotates on its axis. ms can take one of two values, $ \ pm $ 1/2.
Therefore, for a given n, there may be 2n2 areas with different values of l, ml, and ms.
Historically, the primary quantum n label is called a Shell, and n = 1,2,3, ... the shell is sometimes called the K, L, M, ... shell.
The number of orbital quantum l labels subshells, and l = 0,1,2,3,4, ... the subshell is also called s, p, d, f, ... subshell.
If we consider starting electrons to form different atoms, then one would expect all the electrons to enter the lowest energy state, namely n = 1 and l = 0 = ml. This does not happen in nature, however. Paul explained this by posting that the electrons adhere to what is now called the Pauli emission system:
No two electrons in a system can have the same sets of quantum numbers.
Some Solved Examples
Question 1: The kinetic energy of an electron in the second Bohr orbit of a hydrogen atom is equal to $\frac{h^2}{x m a_0^2}$. The value of $2 x$ is
1) 422
2) 528
3) (correct) 631
4) 122
Solution:
$\begin{aligned}
& \frac{h^2}{x m a_0^2} \\
& x=\frac{8 \pi^2 \times 16}{4}=32 \pi^2 \\
& x=32(3.14)^2 \\
& x=315.5 \\
& 2 x=2 \times 315.5 \\
& =631
\end{aligned}$
Hence, the answer is the option (3).
Question 2: The radius of a gold nucleus is $7.24 \times 10^{-15} \mathrm{~m}$. Calculate the volume of the nucleus.
1) $1.15 \times 10^{-44} \mathrm{~m}^3$
2) (correct) $2.81 \times 10^{-44} m^3$
3)$1.52 \times 10^{-44} \mathrm{~m}^3$
4) $3.45 \times 10^{-44} \mathrm{~m}^3$
Solution:
The volume of a sphere is given by the formula $V=\frac{4}{3} \pi r^3$, where $r$, where r is the radius of the sphere. Substituting the given value of the radius, we get: $V=\frac{4}{3} \times \pi \times\left(7.24 \times 10^{-15}\right)^3=2.81 \times 10^{-44} \mathrm{~m}^3$
Therefore, the volume of the gold nucleus is $2.81 \times 10^{-44} \mathrm{~m}^3$.
Hence, the answer is the option (1).
Question 3: The ratio of the energy of the electron in the ground state of hydrogen to the electron in the first excited state of $B e^{3+}$ is
1) (correct) 1:4
2) 1:8
3) 1:16
4) 16:1
Solution:
As we learnt in
Total energy of electron in nth orbit -
$E_n=-13.6 \frac{z^2}{n^2} \mathrm{eV}$
Where $z$ is atomic number
We know that total energy of electron in nth orbit, $E_n=-13.6 \frac{z^2}{n^2} \mathrm{eV}$
$E_1(\text { ground state of hydrogen })=-13.6 \times \frac{1}{1} \mathrm{eV}$
$\mathrm{E}_2$ (first excited state of $\mathrm{Be}^{3+}$ ) $=-13.6 \times \frac{4 \times 4}{2 \times 2}$
$(n=2, z=4)$
$=-13.6 \times 4 \mathrm{eV}$
$\therefore E_1: E_2=1: 4$
Hence, the correct answer is option (1)
Question 4: If the number of revolutions made by electron in 1.0 s in H atom in its $n^{\text {th }}$ orbit is twice of the number of revolutions made by electron in 1.0 s in the $2^{\text {nd }}$ orbit of $\mathrm{He}^{+}$ion atom, then $n$ is
1) (correct) 01
2) 02
3) 03
4) 04
Solution:
(a)
$\begin{aligned}
& \text { no. of revolution }=\frac{V}{2 \pi r}=\frac{v_0 z}{n \times 2 \pi \frac{n^2 a_0}{z}}=N \\
& \Rightarrow N=\frac{V_0}{2 \pi a_0}\left(\frac{z^2}{n^3}\right)=\frac{k z^2}{n^3}
\end{aligned}$
For $\mathrm{He}^{+}$in 2 nd orbit $\mathrm{N}_2=\frac{\mathrm{k}(2)^2}{2^3}=\frac{k}{2}$
$\begin{aligned}
& \Rightarrow 2 N_2=k=N_1 \text { (no of revolutions in H-atom) } \\
& \Rightarrow N_1=k=\frac{k}{n^3} \Rightarrow n=1
\end{aligned}$
Hence, the answer is the option (1).
Also read -
| NCERT Solutions for Class 11 Chemistry | NCERT notes Class 11 Chemistry |
| NCERT notes Class 12 Chemistry | |
| NCERT Notes For All Subjects |
Frequently Asked Questions (FAQs)
Energy levels are discrete states of energy that electrons can occupy in an atom. In the Bohr model, these levels are defined by the electron's distance from the nucleus. The closer an electron is to the nucleus, the lower its energy. When an electron absorbs energy, it can move to a higher energy level (excited state), and when it loses energy, it returns to a lower level (ground state), often emitting a photon in the process.
The theory is that electrons in atoms travel around the central nucleus in a circular motion and can only rotate at a different distance from the nucleus in some circular cycles. Such pathways are related to a specific force and are also called energy shells or energy levels.
Bohr was the first to discover that electrons move around the nucleus in different directions and that the properties of elements are determined by the number of electrons in the outer orbit.
The nucleus of the Bohr atomic model holds most of the atomic mass in its protons and neutrons. Electronically charged electrons contribute less in quantity, but are equal to electricity and protons in the nucleus, circling the well-charged nucleus.
Several changes have been introduced to the Bohr model, especially the Sommerfeld or Bohr - Sommerfeld model, which suggested that electrons rotate the nucleus in elliptical lines rather than circular lines in the Bohr model. The Bohr-Sommerfeld program was actually uncooperative, contributing to many controversial issues.
