Adhesion and Cohesion Difference - Definitions, Relationship, FAQs
In our daily lives, we see evidence of adhesiveness and cohesiveness in many facets of our lives. One of the common phenomena in adhesion and cohesion is force. Despite the fact that they have a similar sounding name, these things are commonly referred to by different names. The link between adhesion and cohesion can be described using surface tension, which is an important water property. As for the definitions, adhesion is the propensity of two or more molecules to form a chemical bond. As opposed to this, Cohesion refers to the force that draws similar molecules together.
This Story also Contains
- Cohesive forces
- Adhesive forces
- Relationship between cohesion and adhesion
- Difference between adhesion and cohesion:
Cohesive forces
Hydrogen bonds and van der Waals forces are examples of intermolecular forces that contribute to the bulk feature of liquids, which is their inability to separate. These attraction forces exist specifically between molecules of the same material. For example, rain falls in droplets rather than a fine mist because water has a high degree of cohesion, which binds the molecules of water together securely to create droplets. Due to the molecules' hatred of their environment, this force tends to bring liquid molecules together, resulting in huge clusters.
Also read -
Adhesive forces
The force of attraction between different substances/objects is referred to as the term "adhesive forces". It includes mechanical forces (sticking together) and electrostatic forces (attraction due to opposing charges). Adhesion occurs when a liquid wetting agent adheres to the surface it is resting on. When water is spilled on a clean piece of glass, it spreads out and leaves behind a thin, even film on the surface. The reason for this is adhesive force. Water molecules are pulled out of the spherical shape due to the adhesive forces between water and glass and hold them against the glass's surface, as a result, the attraction between like molecules is avoided.
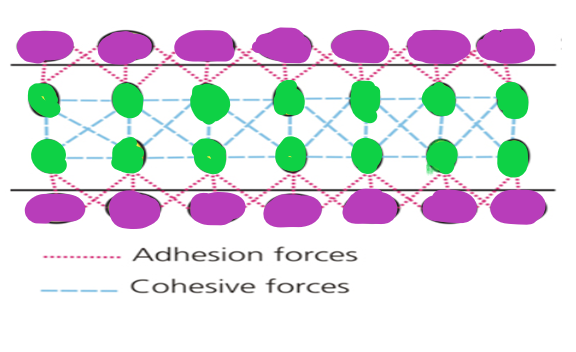
Relationship between cohesion and adhesion
Cohesion and adhesion forces can be found in a variety of activities and processes. As an illustration, consider the meniscus, which is a Both adhesion and cohesion are to blame for liquid surface curvature when kept in a tube or container. In physics, adhesion is the force that pulls liquid against the container wall at its edges. Cohesion is created by the attraction force between water molecules, which causes the liquid's surface to bend in the middle.
The form of the meniscus is also determined by these forces. The meniscus will have a convex form if the adhesion force between the liquid and the inner tube surface is lesser than the cohesion force between the liquid molecules. As an illustration, consider mercury, which is housed in a transparent tube. Similarly, a concave meniscus will result if adhesion is greater than cohesion. For instance, water in a glass tube.
Surface will be parallel when adhesion and cohesion are the same. Assume you've just dropped some water on something. Unless the surface has a high adhesive force, water will run off and become soaked into the fabric. If the cohesive force is strong, water molecules will be more attracted to one another than surface molecules are to one another. As a result, water is absorbed at a slower rate on the surface of the water.
Related Topics Link, |
The macroscopic effects of adhesive and cohesive forces
A liquid's form is defined by the strength of the cohesive and adhesive forces operating on it when it's applied to a smooth surface (and whether or not it will wet the surface).For a given liquid and surface combination, more adhesive forces cause the liquid to sink and saturate the surface beneath it; this is called evaporation. However, if the cohesive forces within the liquid are stronger, the liquid will be able to resist the adhesion and keep its spherical shape, causing it to bead on the surface.
Meniscus is the curvature of a liquid's surface inside a container, like a graduated cylinder.. Be that as it may, we must first understand the adhesive forces acting on surface tension in order to describe why some liquids have a concave up meniscus although others have a concave down meniscus. Water, for example, is a polar molecule with a partial positive charge and partial negative charge on the hydrogens and the oxygen respectively.
As a result, in liquid water, each molecule's partial positive charge attracts the adjacent molecule's partial negative charge. Cohesive forces in water originate here. Because the water molecules are being pulled and pushed equally in all directions, there is no net force on them. As a result, the molecules on the liquid's surface have a net downward pull because they lack upward pushing forces.
The solution lies in the interaction of water molecules' adhesive forces with the container's surface. Liquid convects downward to lessen contact with the wall's surface when its cohesive force is greater than its adhesive force on the wall. When the liquid's adhesive force to the wall exceeds its cohesive force, upward concavity is observed as the liquid is more attracted to the wall than its neighbors.
Also Read:
Difference between adhesion and cohesion:
These two terms refer to forces of attraction that exist between separate molecules and inside the same molecule. Despite the fact that they sound similar, they aren't the same.
Adhesion occurs when two dissimilar molecules or substances come together and stick to one another. A cohesion force is generated when two comparable molecules or substances are attracted by the force of attraction.
Water molecules are attracted to the xylem vessel walls because of adhesion. Within the water molecules, cohesion force is rampant.
The adhesion effects include meniscus and capillary action, as well as (the curved liquid surface in a cylinder). Cohesion has effects on the meniscus, capillary action, and surface tension.
Mechanical or electrical forces generate adhesion between two different substance types. Van der Waals forces and hydrogen bonding cause cohesion.
The liquid can spread all over the surface due to any of the high adhesion forces that are in work there. Water droplets develop on any surface due to a strong cohesive force.
Molecules are drawn to one another by electrostatic attraction. Molecules with similar properties tend to group together.
A liquid's surface tension, water droplets, and capillary action are all caused by surface tension. It's because of the adhesion that a liquid like paint, glue, and cement spreads over a hard surface.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: