Ionization Enthalpy - Meaning, Definition, Trends, FAQs
Ionization enthalpy (or ionization energy) is the energy required to remove one electron from a gaseous atom or ion in its lowest-energy state . Elements with low ionization enthalpy lose their outer electrons easily and tend to form positive ions (cations), making them more chemically reactive. In contrast, elements with high ionization enthalpy hold their electrons tightly, making their ions more stable and their chemical reactions less frequent . Thus, ionization enthalpy is a key measure of how readily an element participates in chemical bonding.
This Story also Contains
- Ionisation Enthalpy
- Ionization Potential
- Factors Affecting Ionisation Enthalpy
- Variation of Ionisation Enthalpy
- Some Solved Examples Based on Ionization Enthalpy
- Conclusion
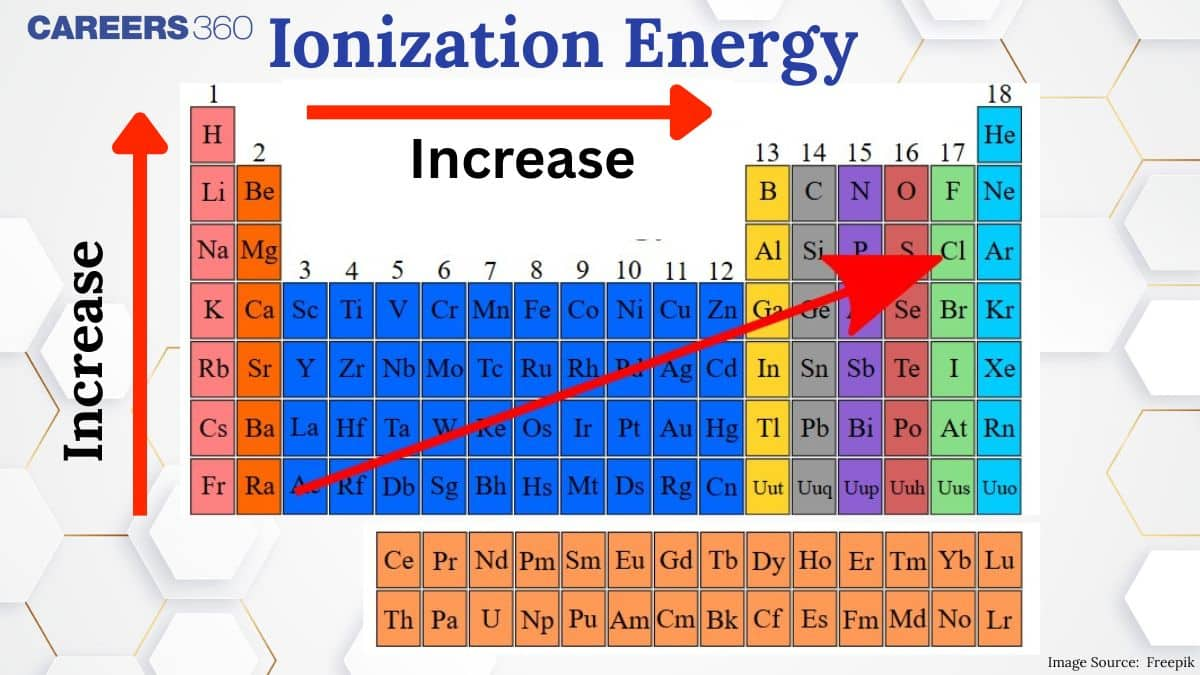
Ionization enthalpy—also known as ionization energy—is the energy required to remove an electron from a gaseous atom in its ground state. It’s a central concept in Class 11 chemistry, frequently tested in board exams and major competitive tests like JEE Main, NEET, BITSAT, and others. Understanding how ionization enthalpy increases across a period (due to stronger nuclear pull) and decreases down a group (due to increased shielding) is vital. Examiners often include 1–2 questions on this topic annually, making it important for scoring well and mastering periodic table trends.
Ionisation Enthalpy
Ionization enthalpy may be defined as the minimum energy required to remove the most loosely bound electron from an isolated gaseous atom to convert it into a gaseous monovalent positive ion.
M(g)→M+(g) + e- (IE1)
IE1 is ionisation enthalpy also known as first ionisation enthalpy.
.jpg)
Ionization Potential
Ionization enthalpy is also expressed in terms of ionization potential. It is the minimum potential difference required to remove the outermost electron from a gaseous atom to form a cation. As the ionisation energy increases, the ionisation potential also increases.

Factors Affecting Ionisation Enthalpy
The ionisation enthalpy of any atom is affected by the following factors.
Size of the atom: The larger the size of an atom, the lower the ionisation enthalpy. As the atomic size increases, the distance between the outermost electrons and the nucleus increases due to which the force of attraction between the nucleus and these outermost electrons decreases, thus it becomes easy to remove an electron from the atom and hence the ionisation enthalpy decreases. Thus,
Ionization enthalpy decreases as Atomic size increases
Screening effect: The higher the value of the screening effect, the lower the ionisation enthalpy. As the screening effect increases, the repulsion between the electrons increases, and thus the removal of an electron from the atom becomes easier. Thus,
Ionization enthalpy decreases as the Screening effect increases
Nuclear charge: As the nuclear charge increases, the force of attraction between the nucleus and electrons also increases and thus the removal of electrons from the atom becomes difficult and hence the ionisation enthalpy increases. Thus,
Ionization enthalpy increases as the Nuclear charge increases
Half-filled and fully-filled orbitals: The atoms with half-filled and filled orbitals are more stable than other atoms. Thus removing an electron from these atoms requires a little more energy. Thus for these atoms with half-filled and fully-filled orbitals, the ionisation enthalpy is higher than others.
The shape of the orbital: The ionization enthalpy also depends on the shape of the orbital in which the last electron enters. The more the orbital is close to the nucleus, the more energy is required to remove the electron in the same orbit. Thus, the ionisation enthalpy for the orbitals from the same orbit follows the given order: s > p > d > f
Variation of Ionisation Enthalpy
When you move down a group in the periodic table, ionization enthalpy drops because new electron shells make the outer electrons farther from the nucleus and less tightly held, making them easier to remove. However, for elements 73 to 82, ionization energy is unusually higher than expected due to the lanthanide contraction—a phenomenon where the added 4f electrons don’t shield the nuclear charge effectively, causing atoms to be smaller and hold their electrons more firmly.

In moving from left to right in a period, the ionisation enthalpy increases. In the period, the nuclear charge increases but the number of shells remains the same, thus the force of attraction between the nucleus and the outer electrons increases, and hence the ionisation enthalpy increases. In a period, some elements like Be, Mg, N, and P have exceptionally higher ionization enthalpies than expected. This is because of their half-filled or filled outer orbital configuration.
For every element, the successive ionisation energy increases. This is because of the increase in the nuclear charge due to the successive removal of electrons.

Importance of Ionisation Enthalpy
Ionization enthalpy is an important factor in determining the nature of an element. The elements with low ionisation enthalpies are metals while the elements with higher ionisation enthalpies are non-metals.
The stability of the oxidation states of an element can also be determined based on the value of ionization enthalpies.
Comparison of IE1 and IE2 of oxygen and nitrogen
Oxygen has an electronic configuration as 1s22s22p4. After IE1, its electronic configuration becomes 1s22s22p3. Now nitrogen has an electronic configuration as 1s22s22p3. After IE1, its electronic configuration becomes 1s22s22p2.
Thus in the case of oxygen, after IE1, O+ has achieved the stable half-filled electronic configuration and hence more energy is required in IE2 to remove an electron further. Similarly, nitrogen already has a stable half-filled electronic configuration, thus more energy is required for IE1 to remove the first electron.
Therefore, the order of different ionization enthalpies is followed as mentioned below:
(i) N(IE1) > O(IE1) (ii) O(IE2) > N(IE2)
Comparison of IE1 and IE2 of chromium and manganese
Chromium has an electronic configuration as [Ar]3d54s1. After IE1, its electronic configuration becomes [Ar]3d5. Now manganese has the electronic configuration as [Ar]3d54s2. After IE1, its electronic configuration becomes [Ar]3d54s1. Thus in the case of chromium, after IE1, Cr+ has achieved the stable half-filled electronic configuration, and hence more energy is required in IE2 to remove an electron further. Similarly, manganese already has stable half-filled d-orbitals and filled 4s orbitals, thus more energy is required for IE1 to remove the first electron.
Therefore, the order of different ionization enthalpies is followed as mentioned below:
(i) Mn(IE1) > Cr(IE1) (ii) Cr(IE2) > Mn(IE2)
Comparison of different ionization enthalpies of N and N+
Nitrogen has an electronic configuration as 1s22s22p3. After IE1, nitrogen becomes N+ and has the electronic configuration 1s22s22p2. Every time some amount of energy has to be supplied to remove the electron. But the nuclear charge remains the same, thus removing the second and third electron from the atom becomes very difficult. Thus for any atom, multiple ionisation enthalpies follow the order given below:
IE3 > IE2 > IE1
Group exception
In moving from top to bottom in a group, the ionisation enthalpy decreases but there are some exceptions as mentioned below.
(i) In group 13, the expected ionization enthalpy is as follows:
B > Al > Ga > In > Tl
But Thallium and Gallium have inner f and inner d electrons respectively due to which there is poor shielding and thus the size reduces and ionisation enthalpy increases. Thus the real order of ionisation enthalpy is:
B > Tl > Ga > Al > In
(ii) In group 14, the expected ionization enthalpy is as follows:
C > Si > Ge > Sn > Pb
But lead(Pb) has inner f-orbitals due to which it has the lanthanoid contraction and thus its size reduces and ionisation enthalpy increases. Thus the real order of ionisation enthalpy is:
C > Si > Ge > Pb > Sn
Also Read:
Recommended video based on ( Ionization Enthalpy)
Some Solved Examples Based on Ionization Enthalpy
Example 1: The increasing order of the first ionization enthalpies of the elements B, P, S, and F (lowest first) is
1) F<S<P<B
2) P<S<B<F
3) B<P<S<F
4) B<S<P<F
Solution: Along a period from left to right, I.E. increases with increasing atomic number, and ionization enthalpy decreases down the group. But P has a half-filled stable configuration as compared to S and because of that ionization enthalpy of P is higher than S. Hence, the answer is option (4).
Example 2: The element having the greatest difference between its first and second ionization energies, is :
1) Ca
2) Sc
3) Ba
4) K
Solution: K is from the first group.
Mg and Sr are from the second group and Sc is from the IIIB group.
K, after IE1 it will reach Noble gas configuration and its IE2 will be very high,
IE2 >>> IE1
Hence, the answer is the option (4).
Example 3: In comparison to boron, beryllium has:
1) lesser nuclear charge and lesser first ionization enthalpy
2) greater nuclear charge and lesser first ionization enthalpy
3) greater nuclear charge and greater first ionization enthalpy
4) lesser nuclear charge and greater first ionization enthalpy
Solution: The variable of effective nuclear charge in the period -
It is observed that the magnitude of effective nuclear charge increases in a period when we move from left to right.
Effective nuclear charge(Zeff) -
Zeff= Total nuclear charge (Z)− screening constant (σ)
"Be" has 4 protons & "B" has 5 protons so, the Nuclear charge of "Be" is less than "B"
Half or fully-filled configurations are more stable than partially-filled orbitals.
IE1 --- Be > B
2s2 2p1
Hence, the answer is the option (4).
Example 4: The formation of the oxide ion O2−(g) requires first an exothermic and then an endothermic step as shown below.
O(g) +e− =O− (g); ΔH∘ = −142kJmol−1
O−(g) + e− =O2−(g); ΔH∘ = 844kJmol−1
This is because
1) oxygen is more electronegative
2) oxygen has a high electron affinity
3) O−ion will tend to resist the addition of another electron
4) O−ion has a comparatively larger size than the oxygen atom
Solution: successive ionization enthalpies.
△iH1 < △iH2 < △iH3
and so on.
As we observed before, successive ionization energies are higher. Similarly, successive electron gain enthalpies are higher.
That is because it is harder to gain another electron OΘ which is already negatively charged.
Hence, the answer is the option (3).
Example 5: Which of the following elements has the lowest value of IE1?
1) Sn
2) Si
3) Pb
4) Ge
Solution: Factors affecting I.E -
The shielding or screening effect reduces the force of attraction between the outermost electrons and the nucleus, hence the outermost electrons can be easily removed.
IE ∝ 1 /screening effect
As you move down a group in the periodic table, ionization energy drops because the number of electron shells increases. This added distance from the nucleus and stronger shielding by inner electrons weakens the pull on the outermost electrons, making them easier to remove. In short, more inner-shell electrons mean less energy is needed to remove a valence electron, reducing the overall ionization energy of the element.
I.E of the 14th group follows the order:
C>Si>Ge>Pb>Sn
because of the poor shielding effect of inner electrons, Pb results in lanthanide contraction. Thus, its IE1 is higher than Sn.
Hence, the answer is the option (1).
Practice more Questions from the link given below:
Conclusion
Ionization enthalpy (IE) helps explain why atoms behave differently in reactions. Elements with low IE lose electrons easily, forming positive ions, which makes them reactive and useful in industries like metallurgy and electronics. In contrast, atoms with high IE hold onto their electrons tightly, making them stable and less likely to react. This explains the unreactive nature of noble gases—they have full electron shells and very high IE, so they hardly ever form chemical bonds. Understanding IE is essential for predicting element reactivity and stability across the periodic table.
Frequently Asked Questions (FAQs)
Ionization energy (IE) is the minimum energy needed to remove an electron from a gaseous atom or ion, typically measured in electronvolts (eV) per atom or kilojoules per mole (kJ/mol) for molecules.
Ionization enthalpy refers to the same process described in thermodynamic terms at constant pressure, expressed in kJ/mol—essentially the heat absorbed when an electron is removed from a gaseous atom .
In practice, these terms mean the same thing, with “energy” used more generally and “enthalpy” denoting a specific heat-related context.
Ionization energy is the energy needed to remove an electron from a gaseous atom, forming a positively charged ion; it's always positive because energy goes into the process.
Electron affinity, on the other hand, is the energy change when an electron is added to a gaseous atom, forming a negative ion. It’s typically negative because energy is released .
In summary, ionization energy measures how tightly an atom holds onto electrons, while electron affinity shows how much it wants to gain one.
A) C> Be> B> Li
B) C> B> Be> Li
C) C> B> Li> Be
D) B> C> Be> Li
Ans: C> Be> B> Li
(A) 5x kJ
(B) 36x/5 kJ
(C) 5x/36 kJ
(D) 9x/4 kJ
Ans: 5x/36 kJ
The initial ionization (eV) values of Be and B respectively are:
(A) 8.29,9.32
(B) 9.32,9.32
(C) 8.29,8.29
(D) 9.32,8.29
Ans: 9.32,8.29
A. 54.4eV
B. 108.8NAeV
C. 54.4NAeV
D. 108.8eV
Ans: 108.8NAeV
