Modern Periodic Table - Introduction, Names, Trend, FAQs
The Modern Periodic Table is a fundamental pillar of chemistry, shaped by centuries of scientific advancement. Through the collaborative contributions of researchers around the world, it offers a structured method for understanding elemental properties. Unlike earlier versions, this table is organized by atomic number—the number of protons in an atom’s nucleus—which directly defines an element and its behavior. This organization not only simplifies classification but also uncovers significant patterns and trends, which fuel theoretical progress and practical applications. Despite undergoing refinements over time, the modern periodic table remains an indispensable tool for chemists, educators, and students, enabling in-depth exploration of elements and their unique characteristics.
This Story also Contains
- Modern periodic table: Arrangement of elements in a table?
- LONG FORM / PRESENT FORM OF MODERN PERIODIC TABLE :
- Solved Examples Based On Modern Periodic Table
- Conclusion
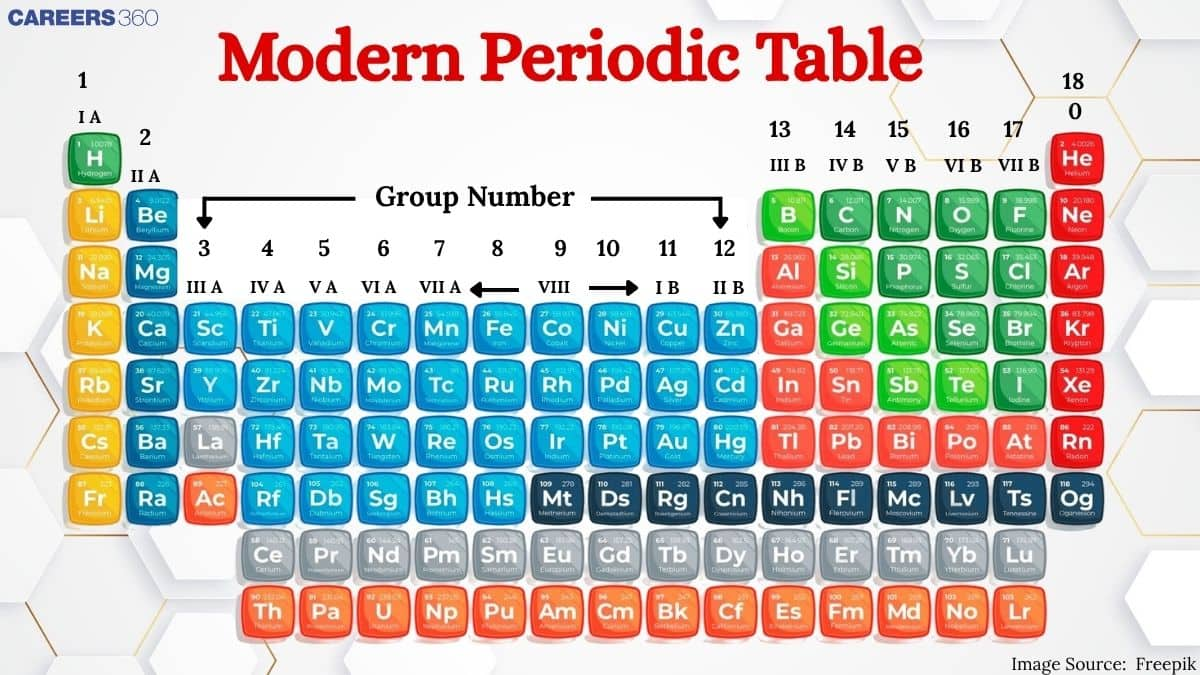
In this article, we’ll explore important facets of the modern periodic table, including “magic numbers,” its long-form structure, and other key aspects. This topic falls under the broader theme of the “Classification of Elements and the Periodic Table,” central to Class 11 chemistry. It’s highly relevant not only for board exams but also for competitive tests like JEE Main, NEET, SRMJEE, BITSAT, WBJEE, BCECE, and others. Over the past decade, NEET has featured questions on this topic, with one appearing in the 2021 exam.
Let’s dive deeper into the modern periodic table, understand its core principles, and work through related problems to strengthen your grasp of the topic.
Also read -
Modern periodic table: Arrangement of elements in a table?
Modern Periodic Table (modified Mendeleev Periodic Table) :
Moseley proposed it.
The modern periodic table is based on the atomic number.
Moseley did an experiment in which he bombarded high-speed electrons on different metal surfaces and obtained X-rays.
He found out that
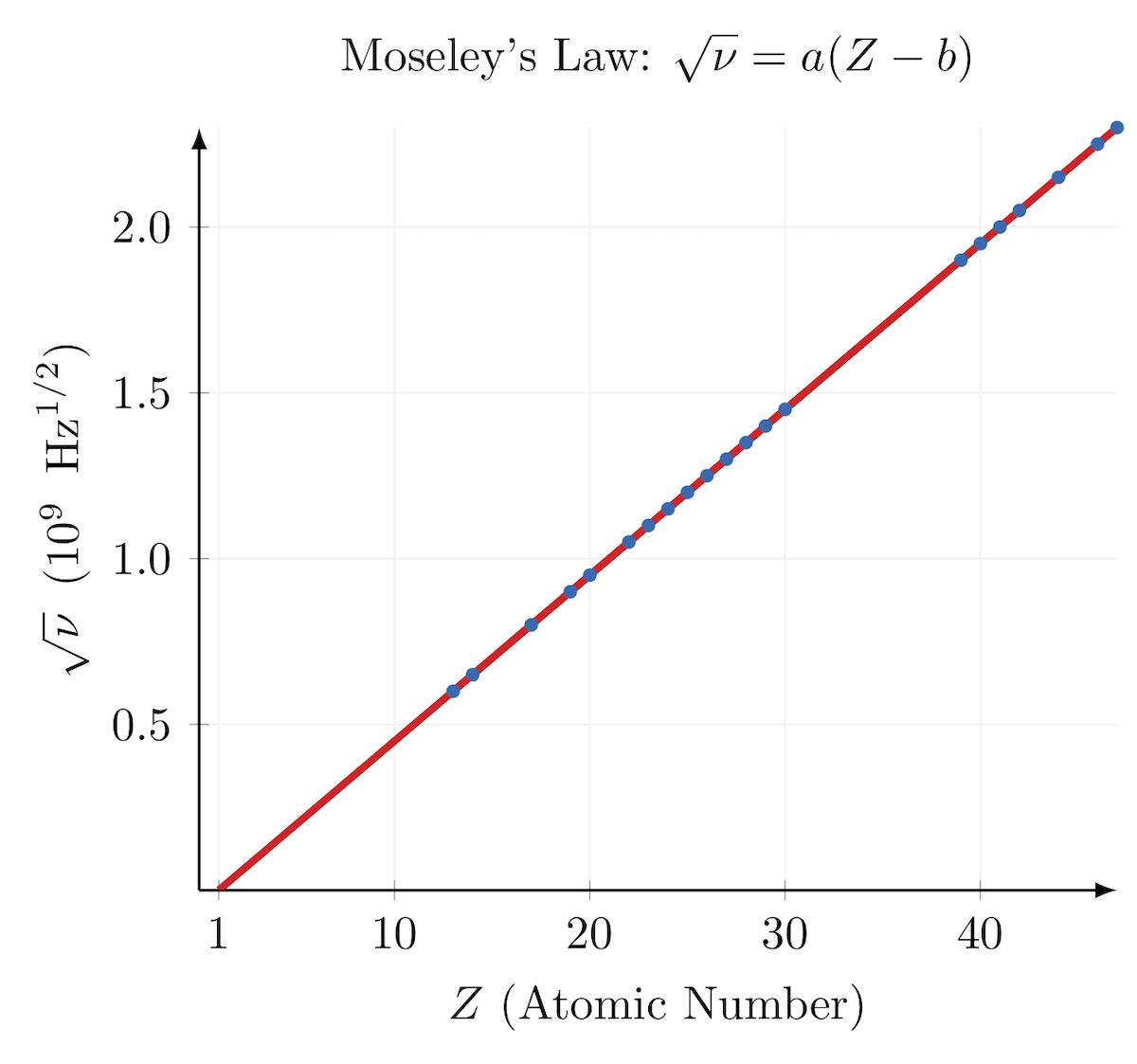
where v = frequency of X-rays, Z = atomic number.
Modern periodic law: The physical & chemical properties of elements are the periodic function of their atomic number.
Characteristics of Modern Periodic Table:
18 vertical columns called groups.
IA to VIIA group, IB to VIIB, and zero group of inert gases.
Inert gases were introduced in the periodic table by Ramsay.
7 horizontal series called periods.
Magic NumberThe magic number of neutrons is the number of neutrons that are present in stable isotopes (non-radioactive). These magic numbers are 2, 8, 20, 28, 50, 82, 126, and 184.
LONG FORM / PRESENT FORM OF MODERN PERIODIC TABLE :
(It is also called as 'Bohr, Bury & Rang, Werner Periodic Table)
It is based on the Bohr-Bury electronic configuration concept and atomic number.
This model is proposed by Rang & Werner
7 periods and 18 vertical columns (groups)
According to I. U. P. A. C. 18 vertical columns are named as Ist to 18th group.
Elements belonging to the same group have the same number of electrons in the outermost shell so their properties are similar.
Elements belonging to the same group have the same no. of electrons in the outermost shell so their properties are similar.
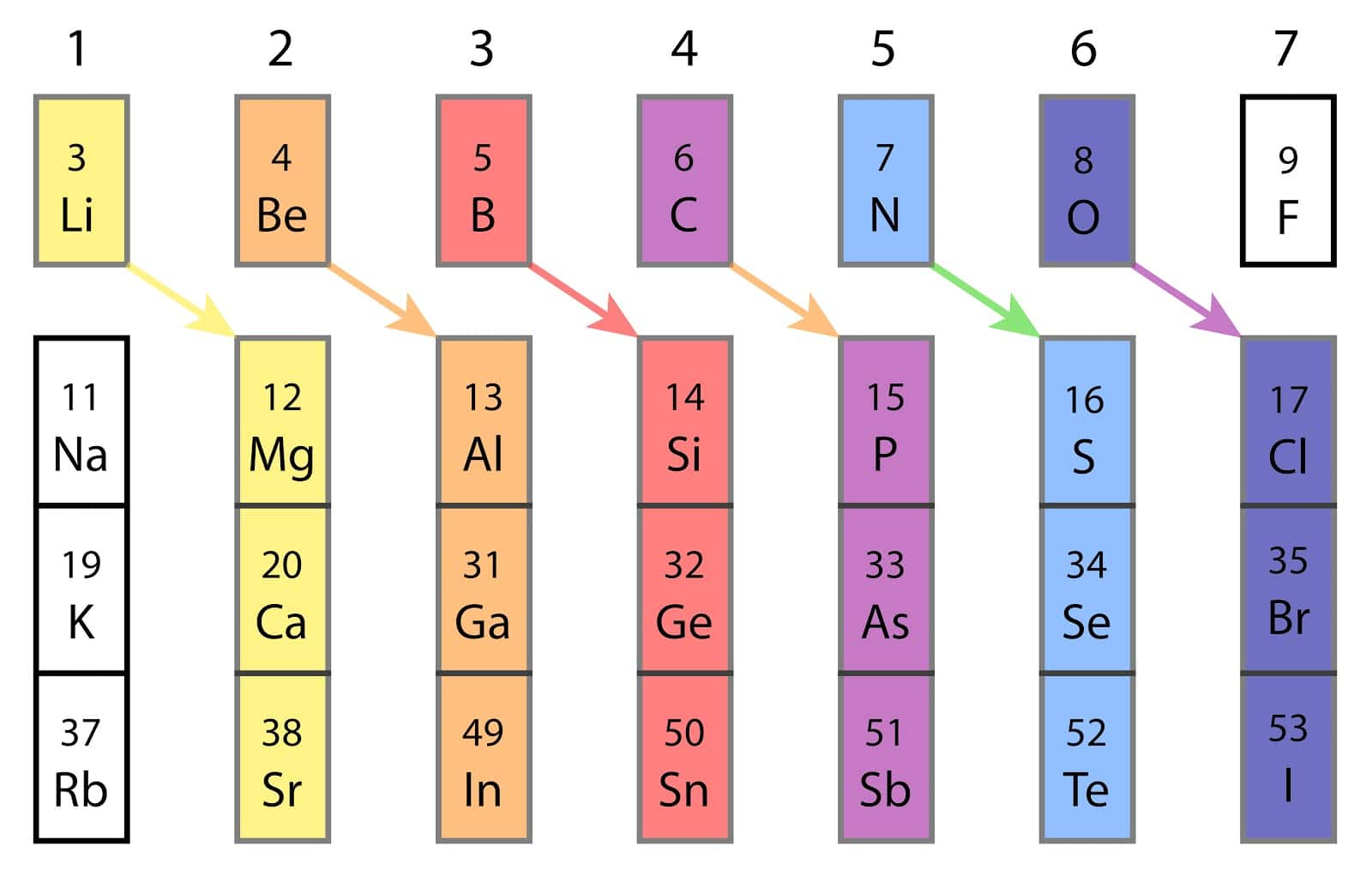
Important Points :Elements in Period 2—lithium (Li), beryllium (Be), and boron (B)—exhibit a distinctive diagonal relationship with certain Period 3 elements (magnesium, aluminum, and silicon). Often called bridge elements, they share chemical traits because their ionic potentials (charge-to-radius ratio) are quite similar—especially notable in the Li–Mg pair, which parallels in charge density and bonding behavior.
The last stable noble gas in the periodic table is radon (Rn), with atomic number 86
Gaseous (11 total): Hydrogen (H), nitrogen (N), oxygen (O), fluorine (F), chlorine (Cl), and the six noble gases—helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
Liquids: Only bromine (Br) and mercury (Hg) are naturally liquid at room temperature, with bromine being the lone nonmetal in this category
Solids: All remaining known elements are solids under normal conditions.
Recommended topic video on (Modern Periodic Table):
Also Read:
Solved Examples Based On Modern Periodic Table
Example 1: The period number in the long form of the periodic table is equal to:
1) Maximum azimuthal quantum number of any element of the period
2) The atomic number of any element of the period
3) Magnetic quantum number of any element of the period
4) Maximum principal quantum number of any element of the period
Solution: The period number in the periodic table corresponds to the principal quantum number of the outermost shell of elements.
Hence, the answer is the option (4).
Example 2: The similarity in chemical properties of the atoms of elements in a group of the Periodic table is most closely related to :
1) atomic numbers
2) atomic masses
3) number of principal energy levels
4) number of valence electrons
Solution: Modern periodic law -
The physical and chemical properties of elements are periodic functions of their atomic number.
Elements that are in the same group, most of the time, have the same number of valence electrons. The number of valence electrons is directly related to the chemical properties of elements.
Hence, the answer is an option (4).
Example 3: According to the periodic law of elements, the variation in properties of elements is related to their
1) atomic masses
2) nuclear masses
3) atomic numbers
4) nuclear neutron-proton number ratios.
Solution: The physical and chemical properties of elements are periodic functions of their atomic number.
Hence, the answer is the option(3).
Example 4: Which one of these is a magic number?
1) 2
2) 8
3) 18
4) 32
Solution: The magic number of neutrons is those present in stable isotopes (non-radioactive). These magic numbers are 2, 28, 20, 28, 50, 82, 126, and 184.
Therefore, 2 is a magic number.
Hence, the answer is the option (1).
Practice more Questions from the link given below:
Conclusion
The modern Periodic Table represents a monumental breakthrough in chemistry, emerging from centuries of investigation aimed at decoding the fabric of matter. Early practitioners, like alchemists, began by isolating and characterizing substances through observation and rudimentary experimentation. Over time, their collective efforts—including systematic theories, atomic structure insights, and predictive arrangements—culminated in a well-ordered system that reveals how elements interact and transform.
From Mendeleev’s pioneering table in 1869, which arranged 63 known elements by atomic weight and predicted the existence of undiscovered ones, to today's sophisticated layout based on atomic number, the Periodic Table has evolved continuously. This evolution has far-reaching practical impact—beyond theoretical chemistry, it guides innovation across industries like electronics, pharmaceuticals, and environmental science .
Frequently Asked Questions (FAQs)
The table is ordered by atomic number—the count of protons in each element’s nucleus—unlike earlier versions which used atomic mass .
These blocks correspond to the type of atomic orbital being filled:
s-block: Groups 1–2
p-block: Groups 13–18
d-block: Transition metals (Groups 3–12)
f-block: Lanthanides and actinides
There are seven periods (rows) and 18 groups (columns) in the current standard periodic table
It is called the periodic table because of the way the elements are arranged. You'll notice they're in rows and columns. The horizontal rows (which go from left to right) are called 'periods' and the vertical columns (going from up to down) are called 'groups'.
Atomic number. The elements are listed in the Modern Periodic Table according to their atomic number rather than their relative atomic mass. The elements are grouped in the periodic table into rows, termed periods, in order of increasing atomic number. Vertical columns, referred to as groups, in which the elements share comparable characteristics of the Modern Periodic Table.
1. It's based on the atomic number of the elements.
2. The number of valence shells in each period is the same.
3. From left to right, the metallic characteristic decreases.
4. The number of valence electrons in a group is the same.
1. Hydrogen's position is unsatisfactory because its features in Modern Periodic Table are similar to those of both Group 1 and Group 17.
2. Isotopes do not have their own position.
3. Its primary body is unable to accommodate inner transition elements (Lanthanides and Actinides).
Ans: Groups are numbered one to eighteen. The s-block, or hydrogen block, of the periodic table contains two groups (1 and 2) of elements; the d-block, or transition block, contains ten groups (3 through 12); and the p-block, or main block, contains six groups (13 through 18).
